The reactions in which two reactive molecules join to generate a single product molecule are known as addition reactions. Such reactions are more likely to occur in compounds having multiple (double and triple) bonds.On the basis of characteristics of invading species, addition reactions are divided into three groups. Electrophiles, nucleophiles, and free radicals are all examples of electrophiles.
Typical Electrophilic Addition
The following are examples of electrophilic additions to alkenes using reagents:
- Reactions involving dihalo addition: X2
- Hydrohalogenations:HX
- H2O hydration reactions
- Hydrogenations H2
- Oxymercuration reactions: Mercuric: acetate, water,
- The Prins reaction: formaldehyde, water.
- hydroboration-oxidation reactions: diborane.
Electrophilic Addition Reaction
Electrophilic addition reactions are those in which electrophiles are involved. This is a common alkene and alkyne reaction.An electrophilic method, for example, is used to add halogen acids to alkenes.

Halogen Addition Reaction
A halogen addition reaction is a simple organic reaction in which a halogen molecule is added to an alkene functional group’s carbon–carbon double bond.
The halogen addition reaction has the following chemical formula:
C=C + X2 → X−C−C−X
(X stands for the halogens bromine or chlorine, and the solvent in this example may be CH2Cl2 or CCl4). A vicinal dihalide is the product.
This reaction is a combination of halogenation and electrophilic addition.
Reaction Mechanism Of Halogen Addition Reaction
The following is a description of the reaction mechanism for alkene bromination.
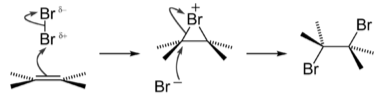
A bromine molecule approaches the electron-rich alkene carbon–carbon double bond in the first phase of the reaction. As the electrons of the double bond resist the electrons of the bromine atom closer to the bond, it gains a partial positive charge.
At this point, the atom is electrophilic and is attacked by the alkene’s pi electrons [carbon–carbon double bond]. It establishes a single sigma bond to both carbon atoms involved for a brief moment. Bromine bonding is unique in this intermediate because, due to its larger size than carbon, the bromide ion can connect with both carbons that earlier shared the -bond, forming a three-membered ring. The bromide ion gains a positive formal charge as a result of this reaction. The halogen ion is now known as a “bromonium ion” or a “chloronium ion,” respectively.
When the first bromine atom attacks the carbon–carbon link, one of its electrons is left behind with the second bromine it was bound to in Br2. That second atom is now a negative bromide anion, attracted to the carbon atoms’ tiny positive charge. The initial bromine atom blocks nucleophilic assault on one side of the carbon chain, so it can only attack from the other side. The link between the first bromine atom and the other carbon atoms breaks when it attacks and makes a bond with one of the carbons, leaving each carbon atom with a halogen substituent.
β-Halocarbocations
The reactive intermediate in an alternate chemical scheme illustrated below is a β-bromocarbocation or β-bromo carbonium ion with one of the carbon atoms being a real carbocation.
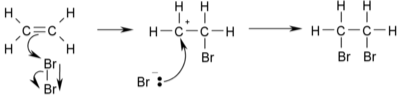
Stereospecificity is not expected or found in reactions that occur through this pathway.The fact that brominations with the maleate ion resulted in cis-addition due to repulsion between the negatively charged carboxylic acid anions being stronger than halonium ion production was already recognised by Roberts and Kimball in 1937. The substituents in alkenes like anetholes and stilbenes can stabilise the carbocation by donating electrons at the expense of the halonium ion.
Hydrohalogenation
The electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to produce haloalkanes is known as a hydrohalogenation reaction.
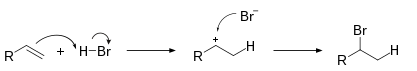
The halogen is found preferentially at the carbon with fewer hydrogen substituents when the two carbon atoms at the double bond are attached to a differing number of hydrogen atoms, according to Markovnikov’s rule. This is due to the alkene removing a hydrogen atom from the acid (HX) in order to generate the most stable carbocation (relative stability: 3°>2°>1°>methyl) while also producing a halogen anion.
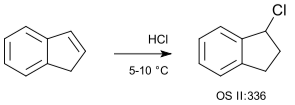
Indene with hydrogen chloride gas (no solvent) is a basic example of a hydrochlorination:
Hydrohalogenation reactions occur in alkynes as well. Depending on the substrate, alkyne hydrohalogenation can be accomplished by a concerted protonation/nucleophilic assault (AdE3) or by protonating the alkyne to form a vinyl cation, then attacking it with HX/X− to produce the product (AdE2). The relative ability of the carbon atoms to stabilise positive charge determines regioselectivity, just as it does for alkenes (either a partial charge in the case of a concerted transition state or a full formal charge for a discrete vinyl cation). The major product could be this first created alkenyl halide, or the product of twice hydrohalogenation to form a dihaloalkane, depending on reaction conditions. The gem-dihaloalkane is the most common regioisomer produced.
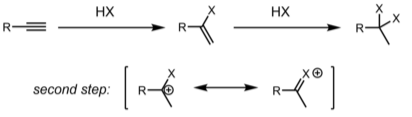
Conclusion
An addition reaction is a reaction in which two molecules combine to generate a bigger one. Nothing is lost in the process. All of the atoms from the smaller molecule are contained in the larger molecule. When an electrophile assaults what we regard to be the “important” molecule, an electrophilic addition process happens. A high-electrons-density location of the “important” molecule is targeted by something with a positive charge.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




