Electronic factors such as electromeric effect, inductive effect, resonance effect, and hyperconjugation influence organic processes. Electronic variables can cause compounds with comparable formula units and structures to respond differently.
With little effort, electrons in a pi (π) bond can be polarized. This effect can be found in organic compounds with at least one multiple bond. The electrons become polarized and shifted towards one of the constituents of the atom when charged reagents like electrophiles or nucleophiles approach them. Electrostatic attraction or repulsion is thTe mechanism at work. The atom that gains the pair of electrons becomes negatively charged, whereas the atom that loses them becomes positively charged.
In simple terms, the electromeric effect occurs when a reagent assaults a bond and moves electrons from one atom to another. The electromeric effect is a transient phenomenon that only occurs in organic molecules.
What is the Electromeric Effect?
The electromeric effect is a molecule polarizability effect caused by intramolecular electron displacement (also known as the ‘conjugative mechanism’ and, historically, the ‘tautomeric mechanism’) in which one electron pair is substituted for another inside the same atomic octet of electrons. As types of electron displacement, the electromeric effect is frequently studied alongside the inductive effect.
Although some individuals refer to it as an effect caused by the presence of a reagent such as an electrophile or a nucleophile, the International Union of Pure and Applied Chemistry (IUPAC) does not define it as such. The phrase “electromagnetic effect” is no longer used in textbooks and is considered obsolete. The words electromeric effect and mesomeric effect imply principles that are absorbed by the phrase resonance effect. Curved arrows, which represent the electron shift, can be used to depict this effect, as shown in the picture below:
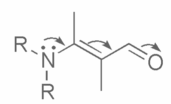
The whole transfer of the shared pair of electrons creates polarity. When the inductive and electromeric effects oppose each other, the inductive effect wins. The inductive effect is the complete transfer of a shared pair of electrons to one of the atoms.
Mechanism of Electromeric Effect
An electromeric reaction’s mechanism can be stated in the following way.
When an electrophile E+ (a reagent) attacks a double or triple bond, the two pi electrons constituting the pi bond are transferred to one atom or the other. The instantaneous development of a dipole in the molecule is caused by the transfer of the shared pi electrons. The following chemical process can be used to depict the electromeric effect.
The displacement of the electron pair is depicted by the curved arrow in this diagram. The atom A loses its portion of the electron pair, which is now held by the atom B. As a result, A receives a positive charge while B receives a negative charge.
Electromeric Effects Are of Various Types
Electromeric effects can be further be divided into two categories:
- The positive electromeric effect, which is also known as the +E effect.
- The negative electromeric effect, which is also known as the -E effect.
Negative Electromeric Effect
When the shared pair of electrons is transported away from the attacking reagent, a negative electromeric effect is observed. This is particularly noticeable in reactions containing carbonyl carbon atoms, such as aldehydes and ketones. Let us take the example of the reaction of an aldehyde with a cyanide ion.
The electrons are transferred away from the attacking reagent and into the system if the attacking reagent is a nucleophile. The –E Effect is what it’s called.
Positive Electromeric Effect
When the shared electron pair is transferred to the attacking reagent, the positive electromeric effect occurs. The attacking reagent creates a connection with the atom that has been given the shared electron pair. The reagent is positively charged in this situation and is looking for a negatively charged site.
The electrons are transported towards the positively charged atom if the attacking species is an electrophile. This is the result of the +E effect.
Conclusion
By understanding the basic concept of the electromeric effect of shared electron pair between the molecules, attacking regent and connection between.
It is characterized by the presence of one electron pair substituted for another inside the same atomic octet of electrons.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




