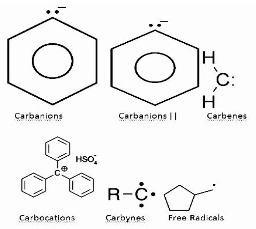
A reactive intermediate, also known as an intermediate, is a short-lived, high-energy, highly reactive molecule in chemistry. It swiftly converts into a more stable molecule when produced in a chemical process. Only severe conditions, such as low temperatures or matrix isolation, can isolate and maintain these molecules. Reactive intermediates can help explain how a chemical reaction works when their presence is indicated.
Definition
In chemistry, a reactive intermediate is a highly reactive, high-energy, short-lived molecule that, when produced in a chemical reaction, swiftly transforms into a stable molecule. They are segregated and stored in some cases. Matrix Isolation and Low Temperatures, for example.
Matrix Isolation is an experimental technique in physics and chemistry that involves a material contained within an unreactive matrix. The host matrix is made up of guest particles that are usually embedded. Molecules, atoms, and ions are examples of guest particles. Within the host matrix, the visitor is isolated.
Reactive intermediates appear in only one of the intermediate steps in some chemical processes, although they present in numerous elementary steps in others. Only quick spectrographic methods distinguish it from a simple chemical intermediate, product, or reactant. The study of how electromagnetic radiation interacts with matter is known as spectroscopy. They are classified based on the type of radiative energy employed in the encounter.
Features of Reactive Intermediates
Chemical trapping can be used to demonstrate its existence. As seen here, a chemical trap is just a chemical compound that recognizes a molecule in specified settings:
- When a molecule is extremely reactive or cannot be determined using spectroscopic methods.
- When the concentration of a molecule is below the detection limit.
- a molecule is present in a mixture and the identification of that molecule is hampered by the presence of other components.
It’s difficult to tell the difference between this and a transition state. It’s a state that has a reaction coordinate and correlates to potential energy at a higher level. It’s a form of chemical reaction in which a certain configuration exists along a reaction coordinate.
Cage effects aren’t taken into account in this case. The cage effect in chemistry depicts how molecule characteristics are influenced by their environment. To interact with other molecules, the cage particle must disperse from its solvent cage.
Allylic Rearrangement
An organic reaction in which the double bond in an allyl chemical molecule changes to the next carbon atom is known as an allylic rearrangement or allylic shift. It occurs as a result of nucleophilic substitution.
The intermediate in reaction conditions that promote an SN1 reaction mechanism is a carbocation, which can have a variety of resonance configurations. After recombination with nucleophile Y, this explains the product distribution (or product spread). An SN1’ substitution is the name for this type of procedure.
Alternatively, in a process known as SN2’ substitution, a nucleophile can attack directly at the allylic site, displacing the leaving group in a single step. When the allyl molecule is unimpeded and a powerful nucleophile is applied, this is likely to happen. The results will be identical to those obtained by substituting SN1’. Thus reaction of 1-chloro-2-butene with sodium hydroxide gives a mixture of 2-buten-1-ol and 3-buten-2-ol:
Nonetheless, the OH group on the main atom produces a small product. The secondary 2-methyl-3-buten-2-ol is created in an 85 percent yield when 1-chloro-3-methyl-2-butene is substituted, whilst the main 3-methyl-2-buten-1-ol is produced in a 15% yield.
Bond in One Reaction Mechanism
This is common in allylic compounds with a bulky leaving group in SN2 circumstances or a bulky non-leaving substituent that causes severe steric hindrance, causing conjugate substitution to increase. Depending on whether the reaction follows an SN1-like mechanism or an SN2-like mechanism, this type of reaction is referred to as SN1’ or SN2’. Similar to how SN1’ and SN2’ analogues exist for SN1 and SN2 reactions, SNi also has an analogue, the SNi’, which is useful for reactions involving allylic chemicals and reagents like SOCl₂.
Three Centre Two Electron
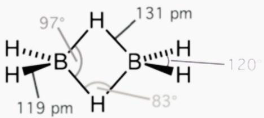
Three atoms share two electrons in a three-centre two-electron (3c–2e) bond, which is an electron-deficient chemical bond. Three molecular orbitals are formed by combining three atomic orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons enter the bonding orbital, creating a net bonding effect and a chemical link between the three atoms. The bonding orbital is tilted towards two of the three atoms in many typical bonds of this sort, rather than being evenly distributed among all three. The trihydrogen cation H₃⁺) and diborane (B ₂H ₆) are two examples of molecules having 3c–2e bonds. The three atoms in each 3c-2e bond form an angular geometry in these two configurations, resulting in a bent bond.
Other Compounds
Trimethylaluminium, which forms a dimer Al₂(CH₃)₆ with the carbon atoms of two of the methyl groups in bridging positions, exhibits this bonding pattern as well. This type of bond can also be seen in carbon compounds, where it’s known as hyperconjugation, which is another name for asymmetrical three-centre two-electron bonds.
Beryllium
A three-centre two-electron -bond consists of donor-acceptor interactions over the C-Be-C core of a Be(0)-carbene adduct in the first stable subvalent Be complex ever discovered.
Carbocations
Three-centre bond transition states are used in carbocation rearrangement processes. Because the three centre bond configurations have nearly the same energy as carbocations, there is almost no activation energy for these rearrangements, which means they happen at an extremely rapid rate.
Carbonium ions have three-centre two-electron bonds, such as ethanium C₂H₇⁺. The 2-Norbornyl cation is perhaps the most well-known and studied structure of this type.
Boranes and Carboranes
Cluster compounds represented by the polyhedral skeletal electron pair theory, such as boranes and carboranes, use an enhanced version of the 3c–2e bond model substantially. Wade’s criteria state that these molecules must have a totally filled set of bonding molecular orbitals in order to be stable.
Conclusion
Reactive intermediate: An unstable reaction intermediate that reacts quickly and has a short lifetime. A reactive intermediate is not to be mistaken with a transition state; the former is a saddle point on an energy profile, while the latter is an energy maximum. Reactive intermediates can help explain how a chemical reaction works when their presence is indicated. A reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate phases in most chemical processes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




