If the two carbon atoms at the double bond are attached to a differing number of hydrogen atoms, the halogen is located preferentially at the carbon with the fewest hydrogen substituents, according to Markovnikov’s rule. This is due to the alkene removing a hydrogen atom from the acid (HX) to produce the most stable carbocation (relative stability: 3°>2°>1°>methyl) and producing a halogen anion.
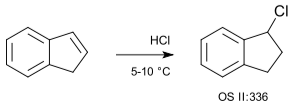
Indene hydrochlorination using hydrogen chloride gas (no solvent) is a basic example of a hydrochlorination:
Anti-Markovnikov Addition
When peroxides are present, HBr adds to a given alkene in an anti-Markovnikov addition pattern. This regiochemistry is the result of the reaction process, which favours the creation of the most stable carbon radical intermediate (relative stability: 3° > 2° > 1°> methyl). The mechanism of this reaction is similar to that of a chain reaction like free radical halogenation, in which the peroxide supports the creation of the bromide radical. In the presence of peroxides, HBr adds so that the bromine atom is added to the carbon with the most hydrogen substituents and hydrogen atoms are added to the carbon with the fewest hydrogen substituents. This process, however, is limited to the addition of HBr.
Other hydrogen halides (HF, HCl, HI) do not act in the same way. The resulting 1-bromoalkanes have a wide range of alkylating properties. They are precursors to fatty tertiary amines by interaction with dimethyl amine. Long-chain alkyl bromides, such as 1-bromododecane, combine with tertiary amines to form quaternary ammonium ions, which are utilised as phase transfer catalysts.
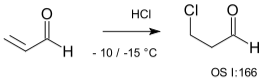
With Michael acceptors, the addition is also anti-Markovnikov because a nucleophilic X now reacts in a nucleophilic conjugate addition, as in the HCl-acrolein reaction.
Hydrogen Chloride
Hydrogen chloride is a diatomic molecule made up of a hydrogen atom H and a chlorine atom Cl held together by a polar covalent bond. Because the chlorine atom is much more electronegative than the hydrogen atom, this bond is polar. As a result, the molecule possesses a high dipole moment, with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. Because of its strong polarity, HCl is highly soluble in water (and in other polar solvents).
When H2O and HCl come into contact, they mix to generate hydronium cations H3O+ and chloride anions Cl via a reversible chemical reaction:
HCl + H2O → H3O+ + Cl−
The resulting solution is a powerful acid known as hydrochloric acid. Because the acid dissociation or ionisation constant, Ka, is big, HCl dissociates or ionises almost completely in water. Hydrogen chloride can behave as an acid even in the absence of water. Hydrogen chloride, for example, can dissolve in some other solvents such as methanol:
HCl + CH3OH → CH3O+H2 + Cl−
Hydrogen chloride can protonate molecules or ions and can also operate as an acid-catalyst in chemical reactions that need anhydrous (water-free) conditions.
Hydrogen chloride is a corrosive chemical due to its acidic nature, especially in the presence of moisture.
Properties of Hydrogen Chloride
- Hydrogen chloride is a colourless gas with a strong odour.
- Because of its high solubility, the gas stinks in damp air.
- In dilute liquids, dissociation is nearly complete. As a result, hydrochloric acid is a highly potent acid.
- Aqueous hydrogen chloride solution is hydrochloric acid.
- It liquefies at 189 degrees Celsius to produce a colourless liquid and freezes at 159 degrees Celsius to form a white solid.
- In the presence of glass, hydrochloric acid is non-corrosive.
- Hydrochloric acid is particularly corrosive, causing metals such as platinum, gold, silver, mercury, tantalum, and others to corrode.
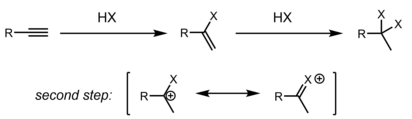
Hydrohalogenation of Alkynes
Alkynes are also subjected to hydrohalogenation reactions. Depending on the substrate, alkyne hydrohalogenation can be accomplished through a concerted protonation/nucleophilic assault (AdE3) or stepwise by first protonation of the alkyne to generate a vinyl cation, followed by HX/X− attack to get the product (AdE2) .The relative ability of the carbon atoms to stabilise positive charge determines regioselectivity, just as it does for alkenes (either a partial charge in the case of a concerted transition state or a full formal charge for a discrete vinyl cation). Depending on the conditions of the reaction, the major result might be this first generated alkenyl halide or the product of twice hydrohalogenation to form a dihaloalkane. The gem-dihaloalkane is the most common regioisomer produced.The resonant stabilisation of an adjacent carbocation by a lone pair on the first implanted halogen explains this regioselectivity. It may be difficult to stop at the first stage, depending on the relative speeds of the two stages, and combinations of the mono and bis hydrohalogenation products are frequently formed.
Conclusion
The Hydrohalogen is a molecule made up of H-X (where ‘X’ represents a halogen). Because halides are very electronegative, when one is attached to a hydrogen atom, the electrons are shared unequally, causing the halogen to draw the majority of the electron density towards itself and away from the hydrogen atom.When electrons cluster around the halogen, the atom acquires a partial negative charge. As a result of having its electrons dragged away, the hydrogen atom will have a partially exposed nucleus. As a result, the hydrogen has a somewhat positive charge.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




