An orbital generated by the combination of two or more atomic orbitals is known as a hybrid orbital. The final orbital has a different shape and energy than the orbitals that make it up . Hybridization is a mathematical technique for explaining atomic bonding and simulating molecular geometry. The VSEPR model predicts geometries that are strikingly similar to those found in actual molecules. Hybrid orbitals are described in a variety of ways.
Hybrid Orbitals Of Carbon
Diamond crystals, like the one pictured here, are prized by practically everyone for their hardness, radiance, and great value. They can also be used in a variety of technological applications. Diamonds, on the other hand, are made up entirely of carbon atoms, with the exception of impurities. Carbon chemistry, like diamond chemistry, is both fascinating and valuable.
With sp , sp2, and sp3 hybrid orbitals, carbon atoms can link to themselves and to other atoms. Organic chemicals are those that have carbon-hydrogen bonds. C−C, C=C, C≡C, C−N, C=N, C≡N, C−O, and C=O bonds Carbon’s capacity to utilize sp , sp2 , and sp3 hybrid orbitals for bonding allows for such diversity. Carbon is also present in inorganic molecules such as carbon monoxide, carbon dioxide, calcium carbonate, sodium bicarbonate, and others.
Types Of Hybridization In Carbon
There are three types of hybridization for carbon –
sp Hybridization
The two sigma bonds around the carbon are linear when sp hybrid orbitals are employed for the sigma bond. Other p orbitals are possible for pi bonding, and acetylene or ethyne HCCH is a common example. It’s worth noting that the molecules HCCH, HCN, and CO all have the same number of electrons. The same idea can explain the bonding in these molecules, therefore their production is unsurprising. The O=C=O molecule is linear, and the carbon atom in this molecule has sp hybrid orbitals as well. This basic molecule similarly has two pi bonds.
sp2 Hybridization
When carbon atoms make use of sp2 hybrid orbitals for sigma bonding, the three bonds lie on the same plane. One such compound is ethene, in which both carbon atoms make use of sp2 hybrid orbitals. One of the remaining p orbitals for each carbon overlap to form a pi bond. A pi bond is made up of two portions, each of which is meant to include bonding electrons.
sp3 Hybridization
For the production of sigma bonds in ethane, the carbon atoms utilize sp3 hybrid orbitals. Each C atom’s four bonds point toward the vertices of a regular tetrahedron, with ideal bond angles of 109.5°. Methane, or CH4, is the most basic chemical and the first member of the alkane family. Ethane, CH3CH3, propane, CH3CH2CH3, butane, CH3CH2CH2CH3, and other members follow.
Types Of Hybrid Orbital
Hybridization can be classified as sp, sp2, sp3, sp3d, sp3d2 based on the types of orbitals involved in mixing.
sp Hybridization
The beryllium atom in a gaseous BeCl2 molecule is an example of a center atom in a linear arrangement of three atoms with no lone pairs of electrons. The two covalent Be–Cl bonds are represented by two valence electron density areas in the BeCl2 molecule. Two of the Be atom’s four valence orbitals will combine to form two hybrid orbitals to accommodate these two electron domains. The valence s orbital is mixed with one of the valence p orbitals in this hybridization process, yielding two equivalent sp hybrid orbitals with a linear geometry.
sp2 Hybridization
A collection of three sp2 hybrid orbitals and one unhybridized p orbital make up the valence orbitals of a central atom surrounded by three zones of electron density. This configuration is the outcome of sp2 hybridization, which involves combining one s orbital with two p orbitals to create three identical hybrid orbitals arranged in a trigonal planar geometry.
sp3 Hybridization
An atom’s valence orbitals are made up of four sp3 hybrid orbitals that are surrounded by a tetrahedral configuration of bonding pairs and lone pairs. The hybrids are created by combining one s orbital with all three p orbitals, resulting in four identical sp3 hybrid orbitals. Each of the tetrahedron’s hybrid orbitals points to a different corner.
sp3d Hybridization
In order to explain the five bonding orbitals in a trigonal bipyramidal configuration, we must employ five valence shell atomic orbitals (the s orbital, three p orbitals, and one d orbital), resulting in five sp3d hybrid orbitals.
sp3d2 Hybridization
To create six sp3d2 hybrid orbitals from an octahedral arrangement of six hybrid orbitals, we must need six valence shell atomic orbitals (the s orbital, three p orbitals, and two of the d orbitals in its valence shell).
Hybrid Orbitals Examples
Example 1:
The carbon.
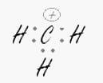
sp2– Trigonal Planar
We only need three hybrid orbitals, also known as sp2, because the carbon has no lone pairs and is connected to three hydrogens.
Example 2:
The oxygen
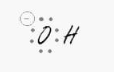
sp3 – Tetrahedral
Remember to account for all of the lone pairings. Every solitary pair requires their own hybrid orbital. For lone pairs, this results in three hybrid orbitals, and the oxygen is bound to one hydrogen, requiring another sp3 orbital. That’s four orbitals, or sp3.
Example 3:
The carbon on the right.
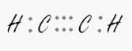
sp – Linear
Because the carbon is connected to two other atoms, it requires two hybrid orbitals, also known as sp.
Conclusion
Hybridization explains not only atom-to-atom bonds, but also molecular morphologies . Hybridization is the process of mixing (hybridizing) two or more separate pure atomic orbitals of the same energy level to produce two or more identical hybrid atomic orbitals.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




