Lewis structures are used in valence bond theory. G.N. Lewis proposed these structures in 1916, based on the assumption that chemical bonds are produced by two shared bonding electrons. In the Heitler-London theory of 1927, quantum mechanics was used to characterize bonding qualities. Using Schrödinger’s wave equation to integrate the wave functions of the two hydrogen atoms, this theory detailed chemical bond formation between hydrogen atoms in the H2 molecule. Linus Pauling proposed valence bond theory in 1928, combining Lewis’s pair bonding notion with the Heitler-London theory. To explain resonance and orbital hybridization, the valence bond theory was devised. In 1931, Pauling released “On the Nature of the Chemical Bond,” a treatise on valence bond theory. The initial computer programmers employed molecular orbital theory to represent chemical bonding, but since the 1980s, valence bond theory principles have been programmable. In terms of properly explaining real behavior, today’s versions of these ideas compete with one another.
Although there is no formal formula for the arrangement of electrons in the atom in chemistry or quantum mechanics, the hydrogen atom can be represented by the Schrödinger equation and the Matrix Mechanics equation, both of which were developed in 1925. However, in 1927, the Heitler–London theory was proposed, allowing for the first time the computation of hydrogen molecule H2 bonding properties based on quantum mechanical considerations. Walter Heitler discovered how to use Schrödinger’s wave equation (1926) to show how two hydrogen atom wavefunctions link together to form a covalent bond, containing plus, minus, and exchange terms. He immediately summoned his associate Fritz London, with whom he spent the night working out the intricacies of the hypothesis.
Later, Linus Pauling combined Lewis’ pair bonding ideas with Heitler–London theory to establish two additional essential concepts in VB theory: resonance and orbital hybridization (1928). (1930). This period represents the beginning of “modern valence bond theory,” as opposed to older valence bond theories, which are essentially electronic theories of valence couched in pre-wave-mechanical terms, according to Charles Coulson, author of the well-known 1952 book Valence.
Linus Pauling’s seminal study on valence bond theory, “On the Nature of the Chemical Bond,” was published in 1931. Based on this essay, Pauling’s textbook On the Nature of the Chemical Bond, published in 1939, has been dubbed the “bible” of modern chemistry by some. This book aided experimental chemists in comprehending quantum theory’s impact on chemistry. The 1959 edition, on the other hand, failed to effectively address the issues that appeared to be better grasped by molecular orbital theory. During the 1960s and 1970s, valence theory’s influence waned as molecular orbital theory became more practical and was applied in huge digital computer programmers. The most challenging challenges of incorporating valence bond theory into computer systems have essentially been overcome since the 1980s, and valence bond theory has had a revival.
The Schrodinger Wave Equation
The Schrödinger equation (sometimes called Schrödinger’s wave equation) is a partial differential equation that uses the wave function to describe the dynamics of quantum mechanical systems. The Schrödinger equation can be used to determine the trajectory, location, and energy of these systems.
The time-dependent Schrödinger equation and the time-independent Schrödinger equation are the two equations which can be considered as-
The time-dependent Schrödinger equation is expressed as follows:
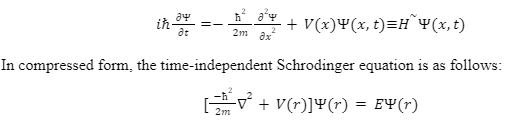
A quantum-mechanical system’s wave function is governed by the Schrödinger equation, which is a linear partial differential equation. It’s a crucial result in quantum physics, and its discovery was a watershed moment in the field’s evolution. The equation is named after Erwin Schrödinger, who proposed it in 1925 and published it in 1926, laying the groundwork for his Nobel Prize-winning work in Physics in 1933.
The Schrödinger equation is the quantum counterpart of Newton’s second law in classical mechanics in terms of concept. Newton’s second law gives a mathematical prediction about the path a given physical system will take over time given a set of known initial conditions. The Schrödinger equation describes the evolution of a wave function over time, which is the quantum-mechanical characterization of a physically isolated system. Because the time-evolution operator must be unitary, the quantum Hamiltonian must be created by the exponential of a self-adjoint operator.
Conclusion
The overlapping atomic orbitals of the interacting atoms form a chemical connection, according to the Valence Bond Theory. Because of the overlapping, electrons are most likely to be in the bond region. Bonds are viewed as weakly connected orbitals in the Valence Bond Theory (small overlap).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




