Because it spreads energy over a greater region rather than keeping it confined to a limited area, charge delocalization is a stabilizing effect. Because electrons are charges, the presence of delocalized electrons adds stability to a system as compared to a system with localized electrons.
Definition
Delocalized electrons in chemistry are electrons that are not associated with a single atom or a covalent bond in a molecule, ion, or solid metal.

Delocalization is a broad concept that can mean different things in different fields
- It refers to resonance in conjugated systems and aromatic molecules in organic chemistry.
- refers to free electrons that aid electrical conduction in solid-state physics.
- It refers to molecular orbital electrons that have spread over numerous nearby atoms in quantum chemistry.
Resonance
The delocalization of six electrons over the C₆ ring in the simple aromatic ring of benzene is commonly visually illustrated by a circle. One sign that the electrons are delocalized is that the six C-C bonds are equidistant; if the structure had isolated double bonds alternating with discrete single bonds, the bond would have alternating greater and shorter lengths. Delocalization in benzene is represented by resonance structures in valence bond theory.
Electrical Conduction
Delocalized electrons can also be seen in solid metal structures. Positive ions (cations) are aligned in a “sea” of delocalized electrons in a metallic framework. This allows electrons to freely travel across the lattice, resulting in qualities like conductivity.
In covalent bonding, all four outside electrons of each carbon atom in a diamond are ‘localized’ between the atoms. Diamond does not carry an electric current because electron mobility is constrained. In graphite, each carbon atom covalently bonds to three other carbon atoms in a plane using only three of its four outer energy level electrons. Each carbon atom provides one electron to the chemical bonding system, which is a delocalized system of electrons. Electrons that have been delocalized are free to travel around the plane. As a result, graphite conducts electricity only in the planes of carbon atoms, not in a direction perpendicular to the plane.
Molecular Orbitals
Standard ab initio quantum chemistry procedures result in delocalized orbitals that, in general, span the entire molecule and have the molecule’s symmetry. Then, using an appropriate unitary transformation, localized orbitals can be found as linear combinations of the delocalized orbitals.
Ab initio calculations demonstrate bonding character in four molecular orbitals in the methane molecule, with electrons shared equally among all five atoms. A bonding molecular orbital created from the 2s orbital on carbon and triply degenerate bonding molecular orbitals formed from each of the 2p orbitals on carbon are the two orbital levels. A linear combination of the four molecular orbitals yields the localized sp³ orbitals corresponding to each individual bond in valence bond theory.
Perchlorate
Perchlorate (ClO₄⁻) is a chemical compound that contains the perchlorate ion. The majority of perchlorates are salts that are manufactured commercially. They’re mostly utilized as oxidizers in pyrotechnic devices and as a static control agent in food packaging. Because of its negative effects on human health, perchlorate contamination in food, water, and other parts of the environment has been researched in the United States. The thyroid gland is sensitive to perchlorate ions.
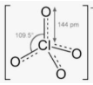
The majority of perchlorates are colorless solids that are water soluble. Ammonium perchlorate NH₄ClO₄, perchloric acid HClO₄, potassium perchlorate KClO₄, and sodium perchlorate NaClO₄ are the four perchlorates of major economic interest. Perchlorate is the anion formed when perchloric acid and its salts dissolve in water and become dissociated. In non-aqueous fluids, many perchlorate salts are soluble.
Production
Perchlorate salts are made industrially by electrolytic oxidation of sodium chlorate aqueous solutions. Sodium perchlorate is made using this approach. The primary use is for rocket fuel. Salts are formed when perchloric acid reacts with bases like ammonium hydroxide. Electrochemically, the extremely valuable ammonium perchlorate can be generated.
Perchlorate can be created by lightning in the presence of chloride, which is unusual. Perchlorate has been found in Florida rain and snow samples, as well as in samples from Lubbock, Texas. It’s also found in Martian soil.
Uses
- Perchlorates are most commonly used as oxidizers in rocket propellants, fireworks, and roadway flares. As a component of solid rocket fuel, ammonium perchlorate composite propellant is particularly valuable. Perchlorates are used extensively in the pyrotechnics business, as well as in certain weapons and the production of matches, in a related but smaller application.
- Perchlorate is used in food packaging to prevent static electricity. When sprayed on containers, it prevents statically charged food from adhering to the surface of plastic or paper/cardboard.
- Lithium perchlorate, which decomposes exothermically to produce oxygen and is used in oxygen “candles” on spaceships, submarines, and other settings where a reliable backup oxygen source is required, is one example.
- Graves’ disease has previously been treated with potassium perchlorate. It obstructs the generation of iodine-containing thyroid hormones.
Carboxylate
The conjugate base of a carboxylic acid, RCOO⁻ (or RCO₂⁻), is called a carboxylate. It’s a negatively charged ion.
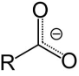
Carboxylate salts have the general formula M(RCOO)ₙ, where n is 1, 2,…, and carboxylate esters have the general formula RCOOR′ (or RCO₂R′). R and R′ are organic groups, with R′ corresponding to the letter H.
Synthesis
Deprotonation of carboxylic acids produces carboxylate ions. Acids with a pKa of less than 5 can be deprotonated by a variety of bases, including sodium hydroxide and sodium bicarbonate.
RCOOH + NaOH → RCOONa + H₂O
Reactions
Nucleophilic Substitution
Ions of carboxylate are excellent nucleophiles. They create esters when they react with alkyl halides. The reaction mechanism is shown in the following reaction.
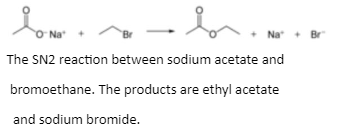
Carboxylate ions have a lower nucleophilicity than hydroxide and alkoxide ions, but are stronger than halide anions (in a polar aprotic solvent, though there are other effects such as solubility of the ion).
Reduction
Due to the lack of a leaving group and the relatively electron-rich carbon atom, the reduction of carboxylate differs from that of ester (due to the negative charge on the oxygen atoms). With a tiny
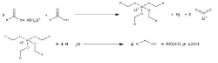
Al–O bonds by turning the LAH into the Lewis acid AlH₃.
Conclusion
Because it spreads energy over a greater region rather than keeping it confined to a limited area, charge delocalization is a stabilizing effect. Because electrons are charges, the presence of delocalized electrons adds stability to a system as compared to a system with localized electrons. Resonance energy is the stabilizing effect of charge and electron delocalization.
Because electron delocalization is induced by conjugation, the more widespread the conjugated system, the more stable the molecule (i.e. the lower its potential energy). If there are positive or negative charges, resonance spreads them out as well.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




