With the chemical formula C6H6, benzene is one of the most well-known aromatic molecules. Benzene is a naturally occurring compound that is found in many plants and animals and is produced by volcanoes and forest fires, but it is also a major industrial chemical made from coal and oil. Benzene is one of the most basic and organic aromatic hydrocarbons, with a large variety of important aromatic compounds as its parent chemical. The substance seems to be a colourless liquid with a distinct odour. This substance is mostly used to make polystyrene. It is reported to be extremely poisonous in nature and is also a carcinogen, meaning that exposure to it can cause leukaemia.
History Of Benzene
Michael Faraday found benzene in lighting gas in the year 1825. In the year 1834, Eilhardt Mitscherlich, a German chemist, heated benzoic acid with lime to generate benzene. Later A.W. von Hofmann, a German chemist, isolated this benzene from coal tar in 1845.
Benzene is a widely used chemical that is found in a variety of things that we use on a daily basis, such as plastics, detergents, and rubber.
Structure Of Benzene
The chemical structure of benzene is depicted in the diagram below. Benzene has the chemical formula C6H6, which means it has six hydrogen atoms and six carbon atoms, with an average mass of around 78.112. The structure has three double bonds and a six-carbon ring, which is represented by a hexagon. A corner that is bonded to other atoms represents the carbon atoms.
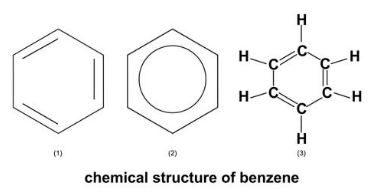
The atoms in benzene are hydrogens. Because the double bonds in this structure are mostly separated by a single bond, it is known as a conjugated double bond arrangement. Inside the hexagon that represents six pi electrons, a circle is used as an alternate sign. All of the carbon-carbon bonds in benzene have the same length of 140pm, according to X-ray diffraction. This figure indicates that the carbon bonds are between the lengths of a single and double bond.
Benzene As Aromatic Hydrocarbon
Benzene is classified as a hydrocarbon due to its chemical formula. It’s a chemical made up entirely of hydrogen and carbon atoms. The formulae and structure reveal benzene to be a pure aromatic hydrocarbon, as well as a ring-shaped compound composed of hydrogen and carbon with alternate double bonds.
Bond Order Of Benzene
The bond order indicates how many chemical bonds exist between two atoms. The bond order expresses the bond’s stability.
Half of the difference between the number of electrons in bonding orbitals and antibonding orbitals is the Bond Order Formula.
There are six molecular π orbitals in benzene. The bond order is equal to half of the difference in bonding and antibonding electrons.
Three of these are bonding, whereas the other three are anti-bonding. The 6 π electrons are distributed among three bonding orbitals.
π BO = ½(B–A) = ½(6–0) = 3
The π bond order for 6 C–C bonds is as follows
For one C–C π bond,
BO = 3/6 = 0.5
In benzene, for a single C–C bond,
Total BO = σ+π = 1 + 0.5 = 1.5
X-Ray Diffraction
X-ray diffraction is a non-destructive solid delineation technique for crystalline materials. It provides data on phases, structures, preferred crystal orientations (texture), and structural factors such as strain, crystallinity, medium grain size, and crystal cracks. The constructive intervention of a monochromatic beam of X-rays dispersed at different angles from each collection of lattice planes in a specimen is used to design XRD peaks. The atomic positions inside the lattice planes define the peak intensities.
As a result, the XRD design in a dispersed substance is the fingerprint of periodic atomic processes. Quick phase identification for a wide range of crystalline samples is possible thanks to a standard library of online research for X-ray powder diffraction patterns. Radiation is dispersed by a regular array of scattering centres with the same spacing as the radiation, resulting in X-ray diffraction. The spacing between diffraction gratings must be equal to the wavelength of diffracted light.
X-Ray Diffraction Analysis
In materials research, X-ray diffraction analysis (XRD) is a technique for determining a material’s crystallographic structure. The X-ray intensities and scattering angles that exit the material are measured using XRD. The identification of materials based on their diffraction pattern is the major application of X-ray diffraction analysis. In phase identification, the x-ray diffraction method also reveals how the ideal structure differs from the exact one due to internal faults and tensions.
Mechanism
X-rays are waves of electromagnetic energy, while crystals are periodic arrays of atoms. The interaction of incident X-rays with the electrons of crystal atoms scatters incident X-rays. Elastic scattering is the name given to this phenomenon, and the electron is the scatterer. The orbicular waves produced by a regular array of scatterers are always the same. These waves cancel each other out in most directions due to destructive interference; however, they combine constructively in less obvious directions, as stated by Bragg’s law
2d sinθ = nλ
The distance between the diffracting planes is denoted by d.
Θ = incident angle
n = integer and
λ = beam wavelength
The specific directions mimic reflections, which are spots on the diffraction pattern. As a result of the electromagnetic waves impinging on a regular array of scatterers, X-ray diffraction patterns form. X-rays are employed to create the X-ray diffraction pattern because their wavelength,, is almost identical to the gap, d, between the crystal surfaces (1-100 angstroms).
X-Ray Diffraction By Crystals
Crystals can diffract X-rays in the same way that a diffraction grating diffracts visible light; in other words, crystals can be utilised as diffraction gratings for diffraction X-rays. This essential concept was first developed by Von Laue in 1912, and it was then tested by Freidrich and Knipping. They proved that an X-ray beam travelling through a single crystal was split into many diffracted beams.
Conclusion
Benzene is a chemical that is commonly used in industry. Benzene can be found in crude oil and is a significant component of gasoline. Plastics, resins, synthetic fibres, rubber lubricants, dyes, detergents, medicines, and pesticides are all made with it. Volcanoes and forest fires naturally create benzene.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




