Butene is an alkene having the formula C4H8 that is also known as butylene. Butene can refer to any of the chemicals alone. They are colorless gases that are found in crude oil as a minor ingredient in proportions that make extraction impossible. Butene is made by catalytic cracking of long-chain hydrocarbons left over from crude oil processing. Fractional distillation is used to extract butene from a mixture of products created by cracking.
Butene can be used as a monomer for polybutene, but it is more costly than polypropylene and other polymers with shorter carbon chains. As a result, polybutene is employed in increasingly specific applications. Copolymers are more often made using butenes (mixed with another monomer such as ethylene).
Properties
At normal temperature and pressure, all four of these isomers are gases, but they may be liquefied by reducing the temperature or increasing the pressure on them, similar to pressurized butane. These gases are colorless but have unique odors, and they are extremely combustible. Although they are not found in substantial concentrations in petroleum, they can be made from petrochemicals or by catalytic cracking. The carbon-carbon double bonds make them more reactive than comparable alkanes, which are more inert molecules in various respects.
These 4-carbon alkenes can function as monomers in the creation of polymers and have additional applications as petrochemical intermediates due to their double bonds. They are used to manufacture synthetic rubber. Isobutylene is a branched alpha-olefin, while but-1-ene is a linear or normal alpha-olefin. But-1-ene, along with other alpha-olefins, is utilized as one of the monomers in the manufacturing of high-density polyethylene and linear low-density polyethylene in a small amount. Butyl rubber is produced via cationic polymerization of isobutylene with 2–7% isoprene. Isobutylene is also used to make methyl tert-butyl ether (MTBE) and isooctane, both of which help gasoline burn more efficiently.
Butene can be used as a monomer for polybutene, but it is more costly than polypropylene and other polymers with shorter carbon chains. As a result, polybutene is employed in increasingly specific applications. Copolymers are more often made using butenes (mixed with another monomer such as ethylene).
2-Butene
But-2-ene has four carbon atoms and is an acyclic alkene. It’s the simplest alkene with cis/trans-isomerism (also known as (E/Z)-isomerism), which means it has two geometric isomers: cis-but-2-ene ((Z)-but-2-ene) and trans-but-2-ene (€-but-2-ene).
It’s a petrochemical made by catalytic cracking of crude oil or ethylene dimerization. Its major applications are in the manufacture of gasoline (petrol) and butadiene, however, some but-2-ene is also used to make the solvent butanone by hydration to 2-butanol and then oxidation.
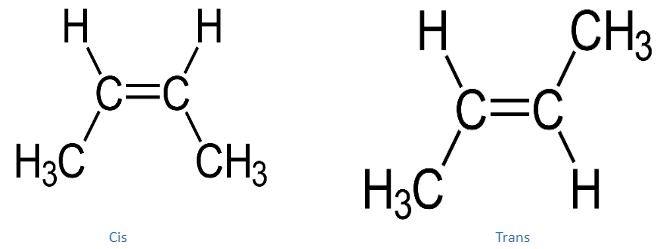
Because of their close boiling points (4 °C for cis and 1 °C for trans), the two isomers are particularly difficult to separate by distillation. In most industrial contexts, however, separation is unnecessary since both isomers behave similarly in most of the needed reactions. 70 percent (Z)-but-2-ene (cis-isomer) and 30% €-but-2-ene make up a typical industrial but-2-ene combination (trans-isomer). Butane and but-1-ene are frequent contaminants that can be found in industrial mixes at 1% or more, along with isobutene, butadiene, and butyne.
Cis-2-Butene
So far, the examples have focused on the simplest organic compounds, the alkanes. Stereoisomers, on the other hand, appear in a variety of different structural kinds of organic chemistry. Two variants of 2-butene, for example, exist in the alkenes. They’re known as cis-2-butene and trans-2-butene, or (Z)- and €-2-butene in more current terminology. The letters Z and E stand for “together” and “apart”. In theory, the structural isomers cis- and trans-2-butene might be interconverted by a simple rotation around the central double bond. The practical reality, however, gets in the way of principle since this rotation would take around 66 kcal/mol of energy, which isn’t accessible under typical circumstances.
Cyclohexane, on the other hand, is highly flexible, as discussed in the section Conformational isomers, with one energy-minimum chair form ring-flipping into another via rotations around carbon-carbon bonds. Consider the isomers cis-1,4-dimethyl cyclohexane and trans-1,4-dimethyl cyclohexane. The second methyl in the trans molecule must be positioned at the equatorial position. What happens, though, if the ring is flipped? Keep in mind that when you flip a ring, all of the axial places become equatorial and vice versa. The equatorial-axial variant of the cis-1,4-dimethylcyclohexane isomer flips into itself, with the axial methyl becoming equatorial and the equatorial methyl becoming axial. As a result, both forms of cis-1,4-dimethylcyclohexane have the same energy.
Conclusion
The atom with the biggest atomic number is given the most priority. Continue along the substituent chain until you find a difference if the atoms directly connected to the double bond are the same. Take, for example, the CH3 and CH2CH3 substituents in 3-methyl-2-pentene. Because it is coupled to two H atoms and a C atom, the carbon atom on the CH2CH3 substituent would be given a greater priority than the carbon atom on the CH3 substituent, which is linked to three H atoms. Determine which carbon atom’s substituent has the most importance. The molecule is defined as the Z isomer if the substituents with the highest priority on both carbon atoms are on the same side of the double bond.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




