Adsorption chromatography can be defined as the process of separating a component from a mixture by adsorption through a stationary component onto a stationary solid surface. Mikhail Tsvet, a Russian botanist, created this method. He utilised this method to separate the pigments chlorophyll and carotenoids. He rinsed a leaf sample with petroleum mixtures after passing it through a wall of calcium carbonate, alumina, and sucrose.
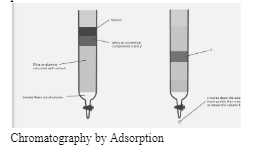
This setup includes an adsorbent that uses Van Der Waal forces and steric interactions between the surfaces to adsorb the solution. This adsorbent is referred to as the solid phase or stationary phase. Depending on the type of chromatography being conducted, the mobile phase is either a gas or a liquid. To elucidate: Gas–solid chromatography uses a gas as the mobile phase, while liquid–solid chromatography uses a liquid as the mobile phase.
ADSORBENT
The adsorbent is a porous material with a large surface area that allows chemicals to be absorbed via intermolecular interactions. Silica gel, cellulose microcrystalline, and other materials are examples. To comprehend the adsorption chromatography procedure, we must first comprehend the numerous components involved in this method –
1.MOBILE PHASE: The mobile phase is either a liquid or a gaseous solvent. It is made to flow through the column, and the solutes are separated from the solvent depending on their compound rate of contact.
2.STATIONARY PHASE: The stationary phase is the adsorbent.
REQUIRED APPARATUS:
The chromatography jar is used to keep the proper temperature for the process to run smoothly.
A thin-layered Borosilicate glass plate with dimensions of 20cm X 20cm, 20cm X 10cm, and 20cm X 5cm is used for chromatography.
The solvent is in the mobile phase (gas or liquid depending on the type of chromatography to be performed)
The adsorbent is in the stationary phase.
FACTORS AFFECTING THE CHROMATOGRAPHY PROCESS:
1.The adsorbent of choice
2.The solvent for the combination is chosen.
3.The pace at which the solvent flows
4.The system’s temperature.
5.For the procedure, the column height.
GOVERNING CONCEPT
The basic principle in this technique is that the Van Der Waal forces and the weak non-ionic force at the surface of interaction allow the adsorbents to hold on to the molecules at their surface. The solutes are separated based on their compound rate of flow when the solvent is passed through the column.
ADSORPTION CHROMATOGRAPHY PROCEDURE:
1.A chromatographic jar that is clean and dry is used.
2.The chromatographic jar’s surroundings is saturated with solvent vapours, and a paper soaked in the mobile phase is affixed to the jar’s walls.
3.After adding the mobile phase to the jar, it is then sealed.
4.The system’s balance is maintained throughout.
5.The adsorbent is designated with a baseline.
6.With the use of a capillary tube, the sample is placed on the TLC plate and allowed to dry.
7.After that, the plates are placed in the jar and the lid is closed.
8.After allowing the solvent to migrate away from the baseline, the TLC plates are removed and allowed to dry.
TYPES OF ADSORPTION CHROMATOGRAPHy
1.Thin-Layered Chromatography: In this approach, the adsorbent is a thin plate, and adsorption occurs as a result of differential migration, which occurs when the solvent flows along the surface of powders distributed on the glass plates.
2.Liquid-Solid Chromatography: this technique employs a liquid as the system’s mobile phase.
3.Gas-Solid Chromatography: in this approach, gas is used as the mobile phase in the setup.
4.Column chromatography: this approach employs the use of a column with adsorbent material filled walls. The solvent is forced through the column, and the solutes are adsorbed in different layers according to their adsorption affinity. The material with the highest affinity is adsorbed at the top of the column, while the material with the lowest affinity is adsorbed at the bottom.
ADSORPTION APPLICATIONS CHROMATOGRAPHY
1.Antibiotics are isolated using this method.
2.It’s used to separate amino acids.
3.This term is used to distinguish between different types of carbohydrates.
4.Fatty acids and fats are identified using this method.
5.Peptides and proteins are isolated using this method.
Conclusion
We conclude that there are numerous applications for adsorption chromatography. It’s typically used to determine a compound’s concentration (or purity), separate a mixture into distinct components, and identify what’s in a combination. Column, thin layer, and gas chromatography are the three primary forms of adsorption chromatography.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




