When it comes to determining the structure of a molecule, the number of electrons involved in a bond is known as bond order. It is used as a gauge of a chemical bond’s stability.The number of bonds between two atoms is a good indicator of bond order. Antibonding orbitals are an exception to this rule.
The equation for determining bond order is as follows:
Bonding electrons are divided by antibonding electrons to determine the bond order.
If the bond order is zero, no bonds exist between the two atoms.In contrast to a compound, elements cannot have a bond order of zero.
Bond Order Examples
Acetylene has a bond order of 3 between its two carbon atoms. Carbon and hydrogen atoms form a one-to-one bond.
Bond Order in Molecular Orbital Theory
According to molecular orbital theory, the bond order can be calculated by dividing the number of bonding electrons by the number of antibonding electrons by two. The valence bond theory makes extensive use of bond order as a measure of bond strength.
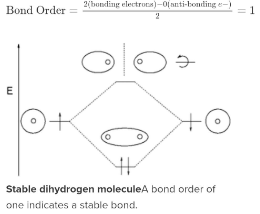
Dihydrogen (H2)
The molecule H2 is depicted in this MO diagram, with the contributing AOs sandwiching the MO. The lower level of bonding has been completely filled. The formula above yields a bond order of one, indicating a stable bond.
Single Bonds
In chemical terminology, a single covalent bond is denoted by a single dash joining two atoms that share an electron pair. The presence of an atom with only one valency is necessary for the formation of this bond. Halogens and hydrogen are examples of single-valency atoms.
Like, for example: C2H6, N3 etc.
In a Lewis structure, a single bond is represented by AA or A-A, where A represents an element. First, each dot represents one shared electron; second, a bar represents both shared electrons in a single bond, as shown. It is the sum of the covalently bound electron pairs between two atoms that determines the bond order. To determine this, draw the molecule’s Lewis structure and count the total number of electron pairs between the relevant atoms.
Single bonds show bond order of 1.
Double Bonds
A double covalent bond is formed when two atoms share two electron pairs between each other, denoted by a double dash joining the atoms. Chalcogens, or members of the oxygen family, are atoms that have two electron pairs.
The two most common gases in the atmosphere are oxygen and carbon dioxide.
Two parallel lines (=) are used typographically to represent a double bond in a skeleton formula between the two connected atoms. Russian chemist Alexander Butlerov was the first to introduce double bonds into the chemical notation system.
It is the sum of the covalently bound electron pairs between two atoms that determines the bond order. To determine this, draw the molecule’s Lewis structure and count the total number of electron pairs between the relevant atoms. The bond order for double bonds is 2.
Triple Bonds
The term “triple covalent bond” refers to the fact that two atoms share three electron pairs. The three dashes connecting the atoms signify a triple bond. Pnictogen atoms, or nitrogen atoms, have triple valency.
N2, C2H2, etc. are examples of compounds.
A triple bond in an alkyne is made up of one sigma bond and two pi bonds. Shaped sp hybrid orbitals direct the two carbon atoms of the triple bond to each other.
It is the sum of the covalently bound electron pairs between two atoms that determines the bond order. To determine this, draw the molecule’s Lewis structure and count the total number of electron pairs between the relevant atoms. The bond order of triple bonds are 3.
Bond Lengths and Strengths
When two nuclei are drawn closer and closer together in order to form a stronger bond, the relationship becomes shorter and shorter. Basically, The coulombic pull on the bonding electrons draws the nuclei closer to each other, resulting in the bond being shorter. At the same time, the greater the amount of pull they exert, the stronger the interaction and bond strength. The bond order is also included in this category. Compared to a single bond between the same two atoms, the strength and length of a double bond are significantly higher and shorter. While a double bond has the advantage of being stronger, it also takes up less space. As you might expect, a single bond is stronger than an order 1.5 bond (such as that found in ozone), but a double bond is weaker. Bond lengths and strengths for all carbon-carbon bonds are shown in the following table.

You might have noticed that the double bond formed by two carbons is not truly twice as strong as a single bond – it’s more like 1.5 times as strong as a single bond, and a triple bond is more like 2.5 times as strong as a single bond. In this example, a triple bond does not have triple strength, but it does have a lot more strength than a single bond. In some cases, the double and triple strengths are greater than the double and triple strengths of the single strength – for example, C–N bonds. As a result, while dealing with strengths, don’t try to read too much into the entire “double” and “triple” thing. Recognize that the strength is in the general vicinity of that location but is not exact.
The lengths of the bonds also differ slightly; a double bond is around 90 percent the length of a single bond, and a triple bond is approximately 80 percent the length of a single bond, respectively. And keep in mind that I’m comparing C-C bonds in this case. When you compare different atoms to different atoms, you will notice that things are a little different. Whatever the case, the trends are present, and you should be aware of the overall trends.
Conclusion
According to molecular orbital theory, the bond order can be calculated by dividing the number of bonding electrons by the number of antibonding electrons by two.When two nuclei are drawn closer and closer together in order to form a stronger bond, the relationship becomes shorter and shorter. The coulombic pull on the bonding electrons draws the nuclei closer to each other, resulting in the bond being shorter. At the same time, the greater the amount of pull they exert, the stronger the interaction and bond strength.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




