Wurtz’s reaction is the organic chemical coupling reaction. In this reaction sodium metal reacts with two alkyl halides in the presence of a dry ether solution which produces a higher alkane as well as the molecule containing sodium and the halogen.
For the synthesis of alkanes, the Wurtz reaction is a particularly useful process in organic chemistry and organometallic chemistry. With the aid of sodium and dry ether solution, two distinct alkyl halides are bonded to generate a longer alkane chain in this process.
This reaction is named after Charles Adolphe Wurtz, a French scientist who also developed the aldol reaction. Metals like silver, indium, activated copper, zinc, and iron, in addition to sodium, can be used in the Wurtz reaction which can produce alkanes..
This reaction uses free radicals as a catalyst, which allows for side reactions that result in the synthesis of alkenes as a by-product. The Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry.
The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents.
Wurtz Reaction Equation
In general form the Wurtz reaction equation can be expressed as follows:
2R-X + 2Na → R-R + 2Na
The two R groups are united in this equation, resulting in an alkane with a longer chain as well as NaX, where X is a Halogen.
Mechanism
The reaction consists of a metal–halogen exchange involving the radical species R and a nucleophilic substitution reaction that forms carbon–carbon bonds.A free radical species designated by R•, which is a component of a halogen-metal exchange, is involved in the mechanism of the Wurtz reaction. The preparation of the Grignard reagents is comparable to this method. Through this reaction mechanism, the carbon-carbon bond is created in a nucleophilic substitution process, which may be broken down into the following steps.
Step 1: When an electron is transferred from a metal (in this example, sodium) to a halogen, an alkyl radical is formed along with the metal halide. The following is an example of a response.
R-X + Na → R• + Na+X
Step 2: A separate sodium atom now contributes a single electron to the alkyl radical, forming an alkyl anion as shown below.
R• + Na → R–Na+
Step 3: The nucleophilic carbon of the alkyl anion displaces the halogen in the alkyl halide through an SN2 reaction and forms a covalent bond with the carbon that was previously linked to the halogen. This step is detailed in the response below.
R–Na+ + R-X → R-R + Na+X–
As previously stated, the free radical mechanism for the Wurtz reaction allows for the production of an alkene as a byproduct. The response presented below explains this side reaction.
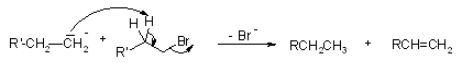
Limitations of The Wurtz Reaction
Because of the potential for negative effects, the Wurtz response is rarely employed. It is used in the synthesis of symmetric alkanes but odd numbered alkanes are restricted. When two different alkyl halides are used as reactants, the result is a mixture of alkanes that is difficult to separate by fractional distillation due to the small changes in boiling points between the products. This process does not produce methane. In the case of tertiary halides, this reaction fails.In addition, because the reaction includes free radical species, an alkene is produced as a byproduct.
This response has a few drawbacks, which are outlined below.
- Because a variety of alkane products is generated when different alkanes are reacted, this approach is often used to synthesize symmetric alkanes (these mixtures are difficult to separate).
- There is a side reaction that produces an alkene product. There is a larger quantity of alkene produced if the alkyl halides are bulky in character, especially at the halogen end.
- Because the result of an organic coupling reaction must include at least two carbon atoms, methane cannot be produced via the Wurtz process.
- When tertiary alkyl halides are utilised, the Wurtz coupling process usually fails.
Uses of Wurtz Reaction
- This reaction is used to close tiny rings, notably those with three members
- The Wurtz coupling is used to make bicyclobutane
- The following is the reaction:
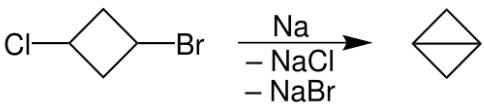
- When the reactant is vicinal dihalide, the product is alkenes, and when the reactant is Geminal dihalide, the product is alkynes
Conclusion
Wurtz coupling, on the other hand, is effective for closing tiny rings, particularly three-membered rings. This method yielded 95 percent bicyclobutane from 1-bromo-3-chlorocyclobutane. The process is carried out in a refluxing dioxane bath, where the sodium is liquid. As a result of numerous product production, this reaction has a low yield. It results in the creation of cyclic products in the case of (1,3), (1,4), (1,5), and (1,6) dihalides. It produces alkenes in vicinal dihalides and alkynes in geminal dihalides.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




