It involves the transformation of many types of matter from one form to another. Different types of reactions cause this. Redox Reactions are one of the most important categories among the numerous types of reactions (Oxidation and Reduction).
Redox reactions come in a variety of forms. To better grasp the classification of redox processes, let’s go over the different types of redox reactions (oxidation and reduction reactions) and their examples.
Redox Reactions Types (Oxidation and Reduction)
Main types of redox reactions can be distinguished:
- Reactions of Combination
- Reactions of Decomposition
- Reactions to Displacement
- Reactions that are out of proportion
1) Combination Reactions
Reactions in which two or more elements are combined. Two species of any atom or molecule unite to form a single species of the chemical in this sort of oxidation and reduction reaction, also known as a redox reaction.
When both species (A & B) or either of the species (A & B) are present in their elemental form, this type of reaction is referred to as a redox reaction. The breakdown process is the polar opposite of this sort of redox reaction (oxidation and reduction).
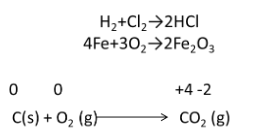
Combustion Reactions
It is a subtype of combination reaction in which one of the species will always be elemental dioxygen.
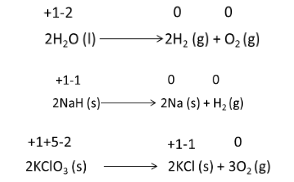
![]() 2) Decomposition Reactions
2) Decomposition Reactions
Combination reactions are the polar opposite of this reaction. A single compound gets broken down into two or more distinct compounds in this sort of reaction. However, at least one of the two or more products must be in its most basic form.
All the above reactions take place under heat as a necessary condition.
Exception:
However, not all the decomposition reactions are redox processes/reactions. For instance:
![]() Metal and non-metal reactivity
Metal and non-metal reactivity
Metals’ Reactivity
Observing the rate of evolution or displacement of hydrogen from dilute/aqueous acids and water can be used to determine the order of reactivity of metals.
The tendency of metals to lose electrons, which indicates the metal’s reducing capacity, can also be used to determine the order of reactivity.
For example, the rate of reactivity of Na is higher than that of Mg. Mg has a higher rate of reactivity than Fe. As a result, Fe reacts the slowest. Furthermore, metals such as silver and gold do not respond in the same way. Metal activity series shows the order of reactivity of metals.
Non-Metallic Reactivity
Non-metals have activity series that are similar to those of metals. Non-metals have an electron accepting propensity, whereas metals have an electron losing tendency, as we all know.
As a result, the oxidising capability of a non-metal determines its reactivity. When considering the halogen group, group number 17’s oxidising power falls from fluorine to iodine.
As a result, fluorine has the greatest oxidising capacity and can displace chlorine, bromine, and iodine from chloride, bromide, and iodide solutions, respectively. F2 has such a strong reactive capacity that it can even displace oxygen from water.
Conclusion
Redox reactions involve the transformation of many types of matter from one form to another. Different types of reactions cause this. Redox Reactions are one of the most important categories among the numerous types of reactions (Oxidation and Reduction).
Redox reactions come in a variety of forms. To better grasp the classification of redox processes, we just studied the different types of redox reactions (oxidation and reduction reactions) and their examples.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





 2)
2)