A resonance form is another technique to represent the Lewis dot structure of a molecule. Lewis structures that are equivalent are referred to as resonance forms. They are utilised when there are multiple possible configurations for double bonds and lone pairs on atoms. When there are multiple ways to build a Lewis dot diagram that fulfills the octet rule, resonance structures form. Keep in mind that the octet rule refers to the process by which an atom earns, loses, or shares electrons in order to have an outer electron shell with eight electrons. They are drawn when a single structure does not accurately represent the true structure.
Resonance structures are a more accurate representation of a Lewis dot structure than Lewis dot structures because they clearly illustrate the bonding between molecules. Not all resonance structures are created equal; some are superior than others. The better ones have the fewest formal charges, the most electronegative atoms have the most formal charges, and the structure maximizes bonding. The more resonance forms a molecule has, the more stable the molecule is. They are connected by a double-headed arrow, indicating that the true structure is between the resonance structures. Curved arrow notation was employed to depict the flow of electrons from one resonance type to the next.
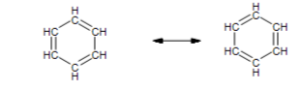
Benzene is a chemical compound that is frequently encountered in Organic Chemistry. It has a resonance structure. Benzene has two resonance structures that illustrate the bond locations.
Bond lengths
When benzene’s two contributing structures are compared, all single and double bonds are swapped. Bond lengths can be determined using a variety of techniques, including X-ray diffraction. A C–C single bond has an average length of 154 pm; a C=C double bond has an average length of 133 pm. The carbon–carbon bonds in localized cyclohexatriene should alternate between 154 and 133 pm. Rather than that, it is discovered that all carbon–carbon bonds in benzene are approximately 139 pm in length, a bond length halfway between single and double bonds. This mixed single- and double-bond (or triple-bond) structure is characteristic of all compounds in which the bond order varies between contributing components. Bond orders can be used to compare bond lengths. For instance, the bond order in cyclohexane is 1, whereas in benzene it is 1 + (3 6) = 1+12. As a result, benzene has a higher proportion of double bonds and thus a shorter bond length than cyclohexane.
Energy of resonance
The resonance (or delocalization) energy is the amount of energy required to transform a really delocalized structure into the most stable contributing structure. The empirical resonance energy can be determined by comparing the enthalpy change of hydrogenation of the actual substance to that of the contributing structure.
The hydrogenation of benzene to cyclohexane is exothermic, requiring 1,3-cyclohexadiene and cyclohexene; 1 mole of benzene produces 208.4 kJ. (49.8 kcal).
![]()
By virtue of resonance, the benzene molecule is stabilized; the pi electrons are delocalized around the ring structure. Each carbon-carbon bond has a bond order of 1.5 as a result of this delocalization, meaning that they are stronger than standard C-C sigma bonds. The delocalization of pi electrons in benzene’s resonance hybrid is described using a circle inside the hexagonal ring.
In benzene, Kekule proposed two cyclohexatriene Kekule structures that, when combined, form the general structure. In the hybrid structure on the right, the hexagon substitutes three double bonds and represents six electrons in a collection of three molecular orbitals with a nodal plane in the molecule plane.
CONCLUSION
When there are multiple ways to build a Lewis dot diagram that fulfills the octet rule, resonance structures form. Keep in mind that the octet rule refers to the process by which an atom earns, loses, or shares electrons in order to have an outer electron shell with eight electrons.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




