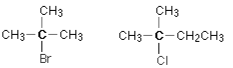The inductive effect in a molecule is a change in electron density produced by electron-withdrawing or electron-donating groups in other parts of the molecule, resulting in a permanent dipole in a connection between the two electrons. In contrast to the electromeric effect found in a (pi) bond, it is present in a (sigma) bond. When the material being examined is nonmagnetic, inductive phenomena such as positive phase angles in impedance measurements have frequently perplexed researchers.
When the measurement is based on the auto-balancing bridge technique and the measured material is a bulky sample, the capacitive coupling from the sample to the ground might cause some of these inductive effects to manifest themselves. The difference in electronegativity of atoms linked together causes the inductive effect. If the electronegativities of two atoms differ, the link between them is polarised. The development of partial charges + and, which have effects on surrounding bonds at a relatively short distance, is caused by the polarisation of the bond.
Body
An example of a distance-dependent phenomenon is the inductive effect, often known as “the -I Effect,” in which the charge of a chemical bond changes the orientation of surrounding bonds in the same or other molecules, leading to a permanent state of polarisation in the molecule. When atoms from two different elements join together to create a bond, the electron density of the bond is not uniformly distributed. When electron clouds form in a bond, they tend to align themselves in the direction of the bond’s more electronegative element.
The inductive effect happens in the molecules of water. The chemical bonds inside a water molecule are more positively charged in the vicinity of the hydrogen atoms, while they are more negatively charged in the vicinity of the oxygen atoms. As a result, water molecules have a polarity. Other factors may quickly counteract the inductive effect, however, due to the small magnitude of the induced charge and the fact that it is only active over short distances.
Alkyl Halides
Haloalkanes are another name for alkyl halides. When working with nonmagnetic materials, inductive effects such as positive phase angles in impedance measurements have frequently caused consternation among the scientists and engineers. Some of these inductive effects can be caused by capacitive coupling from the measured material to ground when the measurement is based on the auto-balancing bridge method and the measured material is a bulky sample, The capacitive coupling between the human body and ground is taken into consideration when designing a model with four electrodes for bioimpedance measurements on the human body. Impedance metres based on an auto-balancing bridge are unable to measure grounded impedances; in order to circumvent this constraint, we shall offer a method for estimating the stray capacitance to ground with these metres in this paper.
Ionisation Constant
An ionisation constant (K) determines the equilibrium between ions and non-ionized molecules in a solution or liquid. So it’s the ratio of products to reactants elevated to stoichiometric powers. In an equilibrium reaction, the backward and forward reaction rates are equal. It is the equilibrium constant for dissociation in acid-base processes. Concentrations, for example, can vary. Because the concentration shows how much material has been removed.
Concentration ratios can then be connected to provide a constant. So there’s more acid and less ions.An effect caused by this inductive effect is the weakening of bonds between heteroatoms (O, N, S…) and hydrogen atoms, which is one of the repercussions of this effect. By decreasing the electron density in the free doublet, an electron-withdrawing group increases the acidity of the O-H bond of an acid (thus weakening the O-H bond), but decreases the basicity of a nitrogen atom in an amine (thus increasing the basicity of nitrogen atom).
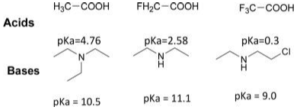
The degree of ion dissociation determines the pH of a substance. K is a technique of linking concentration to other calculations, such pH. For example, reducing acidic pollutants in water prevents system corrosion. Thus, numerous external treatment procedures can lessen water acidity.Any imbalance in the equation can cause corrosion. Also known as a dissociation constant, an ionisation constant Alkyl halides are compounds in which halogen atoms in alkyl halide have replaced one or more hydrogen atoms in an alkane (fluorine, chlorine, bromine or iodine).
Alkyl halides are classified according to how the halogen atom is positioned on the carbon atom chain. Primary, secondary, and tertiary alkyl halides are the three types of alkyl halides. Alkyl halide classifications are commonly used to assist distinguish patterns and trends in the chemical reactivity of alkyl halides. With respect to electronegativity, halogens are more electronegative than carbons. The carbon and halogen always share a single bond since the neutral bonding pattern for halogens is one bond and three lone pairs.
Halides of Primary Alkyls
The carbon connected to the halogen atom in a primary (1°) haloalkane is only coupled to one other alkyl group. The chemicals listed below are examples of primary alkyl halides.
![]()
It’s worth noting that the complexity of the attached alkyl group has no bearing. There is only one bond to an alkyl group from the CH2 group that holds the halogen in each circumstance. Even though there are no alkyl groups linked to the carbon with the halogen on it, CH3Br and the other methyl halides are sometimes counted as primary alkyl halides.
Secondary Alkyl Halides
This carbon is connected directly to two additional alkyl groups, which can be the same or different, in a secondary (2°) haloalkane. These compounds are examples of secondary alkyl halides
Tertiary Alkyl Halides
In a tertiary (3°) halogenoalkane, the halogen is directly linked to three alkyl groups, which can be identical or different. These chemicals are tertiary alkyl halides.
Conclusion
As the name implies, it refers to the situation in which an unbalanced distribution of bonding electrons occurs in any given molecule, causing a permanent dipole to form in the molecule.
The formation of permanent polarisation as a result of the partial displacement of sigma e- along the carbon chain or partial displacement of sigma-bonded electron toward more electronegative atom in the carbon chain (i.e., the magnitude of partial positive charge) is referred to as the sigma effect.
The inductive effect is a long-term phenomenon. This effect can occur in both sigma and pi bonds, but the electromeric effect can occur only in sigma bonds and pi bonds. The influence on electron density in a region of a molecule caused by electron-withdrawing or electron-donating groups in other portions of the molecule is referred to as electron density shift.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out