Electron is the subatomic particle with a negative one elementary charge on its electric charge. Electrons are the first generation of lepton particles and are often considered to be elementary particles due to their lack of known constituents or substructure. The electron weighs approximately 1/1836 the mass of a proton. The electron’s quantum mechanical features include an intrinsic angular momentum (spin) of half-integer magnitude, given in units of the decreased Planck constant. Due to the fact that electrons are fermions, no two can occupy the same quantum state, according to the Pauli exclusion principle. As with all elementary particles, electrons display both particle and wave properties: they can collide with other particles and are diffracted in the same way that light is. Because electrons have a smaller mass and thus a longer de Broglie wavelength for a given energy, their wave characteristics are easier to measure experimentally than those of other particles such as neutrons and protons.
Ozone
Trioxygen, or ozone, is a non-metallic molecule with the chemical formula O3. It is a pale blue gas with a pungent odour. It is an oxygen allotrope that is significantly less stable than the diatomic allotrope O2, decomposing into O2 in the lower atmosphere (dioxygen). Ozone is generated from dioxygen in the Earth’s atmosphere by the action of ultraviolet (UV) light and electrical discharges. It is found in trace amounts throughout the latter, with the highest concentrations in the stratosphere’s ozone layer, which absorbs the majority of the Sun’s ultraviolet (UV) energy.
The odour of ozone is similar to that of chlorine and is discernible by many individuals at quantities as low as 0.1 parts per million (ppm) in the air. In 1865, the O3 structure of ozone was established. The molecule’s structure was eventually shown to be bent and to be slightly diamagnetic. At room temperature, ozone is a pale blue gas that condenses to a dark blue liquid and finally to a violet-black solid at cryogenic temperatures. Due to ozone’s instability in comparison to more abundant dioxygen, both concentrated gas and liquid ozone can dissolve explosively when exposed to high temperatures, physical trauma, or rapid warming to the boiling point. As a result, it is only used commercially in trace amounts.
Ozone is a highly reactive oxidant (much more so than dioxygen) with a wide variety of industrial and consumer applications involving oxidation. However, this same strong oxidising potential leads ozone to harm mucosal and respiratory tissues in animals, as well as plant tissues, at concentrations more than around 0.1 ppm. While this makes ozone a significant respiratory hazard and pollutant near the ground, a higher ozone layer concentration (between two and eight parts per million) is advantageous because it prevents harmful ultraviolet light from reaching the Earth’s surface.
Hypervalent Molecules
A hypervalent molecule is a molecule that contains one or more main group components that appear to have more than eight electrons in their valence shells. Hypervalent compounds include phosphorus pentachloride (PCl5), sulphur hexafluoride (SF6), chlorine trifluoride (ClF3), the chlorite (ClO–2) ion, and the triiodide (I–3) ion.
The ability of an atom in a molecular entity to expand its valence shell outside of the Lewis octet rule. Hypervalent compounds are typical for elements in the second and subsequent rows of the periodic table, groups 14–18. There are two types of hypervalent bonds: one where electrons are moved from one type of atom to another type of atom, and the other where electrons move from one type of atom to another type of atom.
Concept Of Hypervalency In P Block Compounds
A hypervalent molecule is one in which there are more than four pairs of electrons surrounding the core atom in the molecule’s traditional Lewis diagram.
- I. Musher characterised hypervalent molecules in 1969 as those generated by nonmetals in any of their stable valence states more than 3, 2, 1, and 0, respectively.
This is why we call these compounds hypervalent (HV): They have donor atoms that have more valences than the classic theory allows, so they need more electron pairs to bond than the Lewis-Langmuir theory allows. This is why we call these compounds hypervalent. They are called hypervalent molecules because they have the chemical formulas and often the structure of the addition product of a stable molecule and two monovalent ligands or a single divalent ligand. They can also be called hyper molecules
The N-X-L Designation
Using the notation, N-X-L, is a way to refer to hypervalent compounds. In this case, N is the number of valence electrons that can be given to the central atom, X is the central atom’s symbol, and L is the number of ligand/substituents that are directly linked to the atom.The coordination numbers of the compounds range from two to six. All known compounds with rare gases as the core atom are classified as hypervalent molecules. The structure of the majority of hypervalent compounds is derived from a trigonal bipyramid or octahedral geometry.
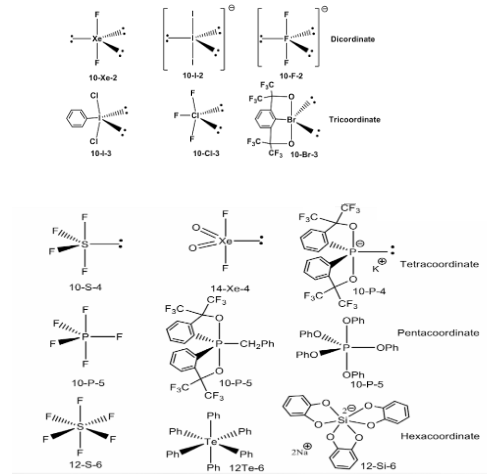
Explaining Hypervalency
Pauling’s expanded octet model
By promoting electrons into unoccupied high-lying d orbitals, sp3d/sp3d2 hybridizations are produced. Numerous theoretical researchers have demonstrated that, even if d orbitals are required to provide quantitative bond energies in hypervalent species, these orbitals have maximum occupancies of 0.3 electrons. In 2013, it was determined that for XeF2, the Valence bond structures associated with the sp3d hybridization model account for only 11.2 percent of the wavefunction and contribute only 7.2 kcal/mol of stabilisation energy, significantly less than the overall binding energy (64.1 kcal/mol).
The discovery of F3– which has the same structure as I3– dealt a major setback to the use of d orbitals to explain the structure of hypervalent molecules, as the central atom of I3 was previously assumed to have a trigonal bipyramidal sp3d geometry, whereas the same is not true for F3, as fluorine is a first-row p block element and thus is not expected to have d orbitals for inducing hybridization.
Conclusion
Electrons are involved in a wide variety of physical processes, including electricity, magnetism, chemistry, and thermal conductivity, as well as gravitational, electromagnetic, and weak interactions. Due to the charge on an electron, it has an electric field surrounding it, and if that electron moves relative to an observer, the observer will see it and generate a magnetic field. According to the Lorentz force law, electromagnetic fields generated by external sources affect the velocity of an electron. When the electrons are accelerated, they absorb or emit energy in the form of the photons. Through the use of electromagnetic fields, laboratory devices are capable of catching both individual electrons and electron plasma. In outer space, special telescopes can detect electron plasma. Electrons are used in a wide variety of applications, including tribology or frictional charging, electrolysis, electrochemistry, battery technologies, welding, electronics, cathode-ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, and gaseous ionisation detectors.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




