Hydrocarbon combustion is the chemical reaction in which a hydrocarbon reacts with oxygen to produce carbon dioxide, water, and heat. Hydrocarbons are organic compounds that include both hydrogen and carbon. They’re well-known for being the primary component of fossil fuels including natural gas, petroleum, and coal.As a result, fossil fuels are commonly referred to as hydrocarbon resources. Energy is extracted from fossil fuels by combusting (burning) them. Despite the presence of contaminants in fossil fuels, hydrocarbon combustion is the principal process of fossil fuel combustion.
As demonstrated in the general process below, combustion with oxygen creates three products carbon dioxide, water, and heat, regardless of the kind of hydrocarbon. The energy required to break the bonds in hydrocarbon molecules is far smaller than the energy required to form the bonds in CO2 and H2O molecules. As a result, a great deal of thermal energy is released during the process (heat).This thermal energy can either be used directly (for example, to heat a home) or transformed to mechanical energy via a heat engine. However, due to efficiency losses, large energy losses (as waste heat) are required, as dictated by the second law of thermodynamics. The resulting usable mechanical energy will be far lower than the hydrocarbon combustion beginning thermal energy.
General Equation of Reaction
- refers to how many carbon atoms are in a hydrocarbon.
- refers to how many hydrogen atoms are present in a hydrocarbon.
- in the hydrocarbon combustion reaction, the amount of oxygen atoms necessary
Chemical Reactions of Alkanes
Alkanes are normally inert to chemical reactions in the absence of a spark or a high-intensity light source. Anyone who has used a match to light a gas burner or dropped a match over lighter fluid-coated charcoal should be aware that alkanes will explode into flame when exposed to a spark. It makes no difference if the initial material is natural gas methane.
Alkanes, like all hydrocarbons, burn in the presence of oxygen to produce carbon dioxide (CO2) and water (H2O) as well as heat.
CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
In disposable cigarette lighters, a combination of butane and isobutane is employed.
2 C4H10(g) + 13 O2(g) 8 CO2(g) + 10 H2O(g)
charcoal lighter fluid has a blend of C5 and C6 hydrocarbons.
C5H12(g) + 8 O2(g) 5 CO2(g) + 6 H2O(g)
or gasoline’s complex blend of C6 to C8 hydrocarbons.
2 C8H18(l) + 25 O2(g) 16 CO2(g) + 18 H2O(g)
These hydrocarbons burn to generate CO2 and H2O after being ignited by a spark, releasing between 45 and 50 kJ of energy per gramme of fuel consumed.
Alkanes react with halogens to generate alkyl halides in the presence of light or at high temperatures. When alkyl chloride reacts with chlorine, it forms an alkyl chloride.
CH4(g) + Cl2(g) → CH3Cl(g) +HCL(g)
When bromine is added, an alkyl bromide is formed.
CH4(g) + Br2(l) → CH3Br(g) + HBr(g)
Chlorine (Cl2) and bromine (Br2) react with alkanes and cycloalkanes by substituting a halogen for one or more hydrogens. Although the processes are exothermic, they require a source of energy to start them, such as ultraviolet light or a high temperature, as in the chlorination of cyclobutene.
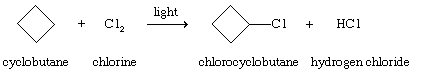
Chemical Reactions of Alkenes and Alkynes
Alkenes and alkynes, which are unsaturated hydrocarbons, are substantially more reactive than their parent alkanes. They react quickly with bromine to add a Br2 molecule across the C=C double bond, for example.
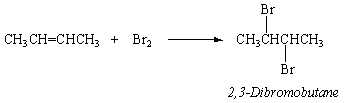
This reaction can be used to detect alkenes and alkynes. Bromine solutions in CCl4 have a bright red-orange colour. There is no change when Br2 in CCl4 is combined with a sample of an alkane at first. When Br2 is combined with an alkene or alkyne, its colour fades quickly.
The addition reaction of 2-butene and bromine to generate 2,3-dibromobutane is just one example of alkene and alkyn addition reactions. To make the equivalent alkyl bromide, hydrogen bromide (HBr) adds across a C=C double bond, with the hydrogen ending up on the carbon atom that had more hydrogen atoms to begin with. When HBr is added to 2-butene, for example, 2-bromobutane is formed.
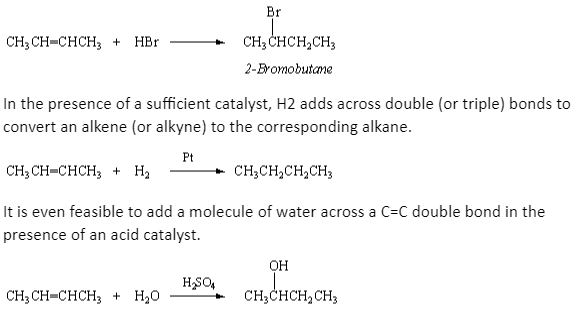
Addition reactions allow new substituents to be added to a hydrocarbon chain, resulting in new derivatives of the parent alkanes.
Conclusion
Hydrocarbon combustion is the chemical reaction in which a hydrocarbon reacts with oxygen to produce carbon dioxide, water, and heat. Hydrocarbons are organic compounds that include both hydrogen and carbon. They’re well-known for being the primary component of fossil fuels including natural gas, petroleum, and coal.
Carbon monoxide is a main contaminant in the troposphere, as it is a by-product of hydrocarbon combustion.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




