When there is a charge separation within a molecule, dipole moments occur. Dipole moments are caused because of the changes in electronegativity and can occur between two ions in an ionic bond or between atoms in a covalent binding. The dipole moment usually grows in proportion to the difference in electronegativity. The size of the dipole moment is c When there is a charge separation within a molecule, dipole moments occur. Dipole moments are caused because of the changes in electronegativity and can occur between two ions in an ionic bond or between atoms in a covalent binding. The dipole moment usually grows in proportion to the difference in electronegativity. The dipole moment is the measurement of a molecule’s polarity within a molecule is determined by the distance between the charge separations. The dipole moment is the measurement of a molecule’s polarity within a molecule.
A dipole moment will be created when electrons are shared unequally among atoms in a molecule. This happens when one atom is more electronegative than another, causing that atom to pull harder on the shared pair of electrons, or we can say when one atom has a lone pair of electrons and the electronegativity vector points in the same direction. A water molecule, which consists of one oxygen atom and the two hydrogen atoms, is one of the most prevalent instances. Oxygen has a partial negative charge, while each hydrogen has a partial positive charge due to differences in electronegativity and lone electrons.
Dipole Moment
An electric dipole is formed when two opposite-sign and equal-magnitude electrical charges are separated by a distance. Dipole moment (μ) is used to determine the size of a dipole. The distance between the charges multiplied by the charge (1 Debye = 3.34*10-30cm) is used to calculate the dipole moment.
The separation of two opposite electrical charges are measured by a dipole moment. And the quantity of the dipole moments is the vector quantity. The charge multiplied by the distance between the charges determines the magnitude, and the direction is from negative to positive charge:
μ = q * r
where,
r = the distance between the charges,
q = the magnitude of the separated charge, and
μ = the dipole moment.
The structure of H2O is bent (by VSEPR theory) because of the lone pair on oxygen, which means that the vectors encoding the dipole moment of each bond do not cancel each other out. As a result, water is polar.
Polarity and Structure of Molecules
The total polarity of a molecule is determined by its shape and the polarity of its links. Depending on the structure, a molecule with polar bonds may or may not have any overall polarity. The simple definition regarding whether a complex molecule is polar or not is if the positive and negative charge centers of the molecule overlap. The molecule has no overall polarity if these centers are located at the same position in space (and is nonpolar). If a molecule is entirely symmetrical in nature, the dipole moment vectors on each molecule cancel out, resulting in a nonpolar molecule. The structure of a molecule can only be polar if it is not symmetrical in nature.
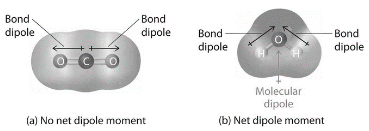
Dipole Moments are only formed when two ions form an ionic link or when two molecules form a covalent bond. The difference in electronegativity of the atoms within the compounds created is the major cause of the rise of the dipole moment. The distance between the bond and the dipole moment is also an important point in determining the amplitude of the dipole moment.
It mainly happens in a similar way with the atom, lone pair of electrons and the difference in the vector points of electronegativity. The water molecule, which consists of one extremely electronegative oxygen atom and two electropositive hydrogen atoms, is one of the most prevalent instances. As a result of the difference in electronegativity and the existence of the lone pair of electrons on the oxygen atom, the oxygen atom has a partial negative charge whereas the hydrogen atom has positive charge.
Significance of the Dipole Moment
Dipole moments are used in chemistry to describe the electron distribution between two linked atoms. The difference between polar and nonpolar bonds is the presence of a dipole moment. Polar molecules are those which have a net dipole moment. The link and molecule can be considered nonpolar if the net dipole moment is 0 or extremely tiny. Atoms which have similar electronegativity levels are more likely to form chemical bonds with low dipole moments.
Conclusion
The bond dipole moment (μ) is a vector quantity with the same direction as the bond axis. An arrow used to indicate dipole moments in chemistry start at the positive charge and stop at the negative charge.
By understanding the concept of the dipole moment we can estimate the character of the molecule and consider it as polar or nonpolar.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




