Amine is a known ammonia derivative in organic chemistry. Chemically, it is a type of organic nitrogen molecule that has a lone pair of electrons in each nitrogen atom it contains. In other words, the hydrogen in amines is replaced by a group of alkyl or aromatic hydrocarbons. Aliphatic and aromatic amines are both types of amines. However, they are classed differently. Additional subgroups are defined by the number of carbon atoms linked to nitrogen, the number of hydrogens replaced and amines.
Types of Amines
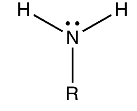
Primary Amines
When one of the three hydrogen atoms in ammonia is replaced with an alkyl or aromatic group, the result is a primary amine. Amino acids and methylamine are examples of primary alkylamines, while aniline is a primary aromatic amine.
Secondary Amines
Two organic substituents (alkyl or aryl or both) are attached to the nitrogen by one hydrogen in secondary amines. Dimethylamine and aromatic Amine, such as diphenylamine, are two examples.
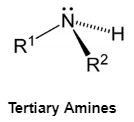
Tertiary Amines
Nitrogen with three organic substituents is known as a tertiary amine. Trimethylamine and EDTA are two examples of tertiary Amines.
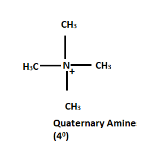
Quaternary amines
The nitrogen of quaternary amines includes four organic substituents, giving them a positive charge.
Cyclic Amines
Another class of amines is known as “cyclic,” which is defined by how the nitrogen-linked substituents connect to each other. Cycles of ammonia can be secondary or tertiary, depending on the structure. Piperidine and aziridine are two examples of six- and three-membered rings.
Structure of Amines
The structure of amines has a nitrogen atom that is sp3-hybridized. The lone pair of electrons in the fourth sp3 hybrid orbital create sigma bonds with three other sp3 hybrid orbitals. The non-bonding lone electron pair points to the vacant corner of a tetrahedron in single-bonded nitrogen’s trigonal pyramidal form.
It is common to categorize the structure of amines into groups based on the settings in which they attach. Primary amines are amines in which an alkyl or aromatic group replaces one of the three hydrogen atoms. Secondary amines have two substituents and one hydrogen linked to a nitrogen atom in their structure. Organic substituents replace the hydrogen atoms in tertiary amines, which are amines with no hydrogen left. As the last point, cyclic amines are those in which the nitrogen has been integrated into a ring structure, effectively making it either secondary or tertiary amine. A nitrogen atom, one electron pair, and three substituents make up the basic structure of an amine. Having four organic substituents on the nitrogen, on the other hand, results in a charged nitrogen center in the form of an ammonium cation.
Characteristics of Amines
- There is an unpleasant fishy smell coming from the lower aliphatic amines.
- At average temperature, primary amines are liquids with three to four carbon atoms; higher amines, on the other hand, are solids.
- Colorless arylamines such as aniline are common. However, they begin to rust when left out in the open.
- Lower aliphatic amines are water-soluble because they form a hydrogen bond with water—amine solubility, and molar weight decreases when the hydrophobic alkyl component rises in size.
- Solvents can’t dissolve the soil’s amines. Organic solvents such as benzene and ether can be employed to dissolve amines.
- Intermolecular interactions frequently involve primary and secondary amines because of the hydrogen and nitrogen interactions with other atoms’ hydrogen.
- Due to two hydrogen atoms in primary amines, intermolecular interactions are more apparent than in secondary amines. In contrast, tertiary amines lack intermolecular interactions due to the lack of free hydrogen atoms in their structure.
- The odor diminishes with the increasing size until it is virtually undetectable. Although this is generally true, there are several exceptions to the rule.
- Amines don’t have any pigment. All quantities of amines with fewer than six carbons can be dissolved in water. Compared to ammonia, aliphatic amine bases are more potent; aromatic amine bases are less so.
Aliphatic vs Aromatic Amines
- The nitrogen in aliphatic amines is intimately linked to alkyl groups and hydrogen atoms, making them highly reactive. Aromatic amines have at least one benzene ring with nitrogen connected directly to it.
- Alkylamines do not have benzene rings directly connected to the Nitrogen atom. At least one benzene ring is linked to the nitrogen atom in aromatic amines. It is only possible to have aromatic rings in aliphatic amines with the direct attachment of one nitrogen and one carbon to the other carbon atom.
- Aromatic amines are heavier than water. However, this is not always the case with aliphatic amines.
- Ultraviolet light cannot penetrate aliphatic amines. On the other hand, aromatic amines absorb strongly at specific wavelengths.
- Compared to aromatic hetero cyclic amines, aliphatic hetero cyclic amines are more potent bases.
- Alkyl ammonium ions are more stable in alkyl ammonium ions than in aromatic ammonium ions. In contrast, all aliphatic amines are weak bases, but they are nonetheless slightly stronger than ammonia.
Final Words
Amine is a nitrogen atom with a lone pair of electrons and three substituted atoms in its general structure. A positive charge on the nitrogen atom can be achieved by binding to four different substituents. It’s possible that these charged species can be used in crucial steps in reactions.
An amine is a primary chemical since it has only one pair of electrons. Compound basicity can be affected by adjacent atoms, steric bulk, and solubility of the cation to be generated. As a result of their ability to form hydrogen bonds, amine compounds are water-soluble and have high boiling temperatures.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out