Sodium bicarbonate is commonly used to treat indigestion. It is also used to treat stomach ulcers, athletic performance, kidney damage, dental plaque, tooth discoloration, and a variety of other conditions, but there is little scientific evidence to back up many of these claims.
Occurrence of NAHCO3:
The mineral nahcolite contains natural sodium hydrogen carbonate. It is the natural source of the compound, and it can also be found in many mineral springs.
Production of Sodium Bicarbonate.
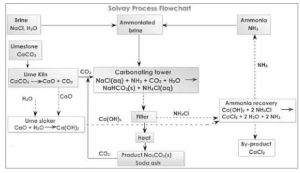
It is produced on a large scale by combining cold, concentrated brine (sodium chloride) solutions with ammonia and carbon dioxide. To make sodium carbonate, the Solvay technique is used (washing soda).
 An example of a reaction is as follows:
An example of a reaction is as follows:
NH4Cl + NaHCO3 = NaCl + H2O + CO2 + NH3
As an intermediate product, this technique generates sodium hydrogen carbonate. Around 373K, it decomposes, releasing carbon dioxide gas and forming sodium carbonate.
Na2CO3 + H2O + CO2 = 2NaHCO3
How does sodium bicarbonate react with water?
Sodium bicarbonate, also known as baking soda, is a weak base that is commonly used in cooking. It ionises weakly in water: No reaction is there in dry powder, but after adding water for cooking, the reaction occurs to release carbon dioxide, causing the baked item to “rise.”The fizzing occurs when baking soda (sodium bicarbonate) and citric acid chemically react in water. They produce sodium citrate, water, and carbon dioxide gas, resulting in bubbles. The baking powder-containing water bubbles up. Baking soda water does not bubble because it is an alkali, the chemical opposite of an acid.When we mix baking powder with water or milk, alkali and acid react and produce carbon dioxide – bubbles.
Effectiveness and ineffectiveness of sodium bicarbonate:
Effectiveness:
- Discomfort (dyspepsia): When taken orally, over-the-counter antacid products containing sodium bicarbonate are thought to be effective for indigestion. They are FDA-approved for this application.
- Athletic ability: Taking sodium bicarbonate orally appears to improve athletic performance by a small amount in the majority of people.
- Toxicity caused by drugs that interfere with the function of sodium channels (sodium channel blockade). Giving sodium bicarbonate intravenously appears to help reduce the side effects of drugs that cause sodium channel blockade.
Ineffectiveness:
- Heart function is suddenly lost (cardiac arrest). Giving sodium bicarbonate intravenously has no effect on survival in children or adults who have had a cardiac arrest outside of the hospital. It may even impede recovery.
- Tissue damage caused by restricted blood flow, followed by restoration of blood flow (ischemia-reperfusion injury). In people who have had heart surgery, IV sodium bicarbonate does not prevent kidney problems. A healthcare provider can only administer IV products.
Medical applications of sodium bicarbonate:
- It is an antacid that is used to treat indigestion and heartburn. Its quick action reduces stomach acid, providing only temporary relief. Its alkaline composition aids in indigestion relief by neutralising excess hydrochloric acid in the stomach.
- A 5% sodium bicarbonate infusion injection is given in medical emergencies such as severe renal failure, a heart attack, or uncontrolled diabetes.
- It is used in the production of beauty care, cosmetics, and personal hygiene products.
- It is used in the manufacture of ear drops.
- It is administered intravenously to alleviate the side effects of chemotherapy.
- It is used to clean the mouth and teeth due to its antibacterial properties.
Characteristics of sodium bicarbonate:
- Powdery condition.
- White crystalline powder in appearance
- The odour is undetectable.
- Under normal temperatures and pressures, it is stable.
- Carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide are all produced during the decomposition process.
- Polymerization that is hazardous will not happen.
- Incompatible with oxidising, reducing acids, alkalis, and moisture.
- A sodium bicarbonate solution has a pH of 8.3.
- When phenolphthalein is used, it does not change colour; however, when methyl orange is used, it turns yellow.
- It is only marginally soluble in water.
- It has a boiling temperature of 8510 degrees Celsius and a melting temperature of 500 degrees Celsius.
Conclusion:
Baking soda, cooking soda, bread soda, and bicarbonate of soda are all names for sodium bicarbonate. It’s widely used in baking and medicine.Carbon, sodium, oxygen, and hydrogen make up sodium bicarbonate.It is weakly basic in nature. Because it has been hydrolyzed, a solution of sodium bicarbonate is mildly alkaline. Baking soda can be used in the garden as a natural pesticide to kill harmful insects and fungi that attack plants.It can be mixed into the soil to increase the pH and make it alkaline. A variety of plants prefer alkaline soils.It can also be used to clean garden tools.Baking soda, as can be studied, is a vital sodium molecule with a specific place in our homes and lives.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




