The bond length in chemistry refers to the equilibrium distance between the nuclei of two linked groups or atoms. There are many different forms of bonds, and the length of each one is determined by what type of atoms are involved. Bonds between atoms might be different depending on the molecule they are part of. Methane, for example, has a carbon-hydrogen bond that is distinct from that of methyl chloride. More electrons in a bond results in a shorter bond. Solid bond lengths can be measured using X-ray diffraction. Gases can be approximated in length by using microwave spectroscopy.
Example of Bond Lengths
Picometers are used to denote the lengths of bonds (pm). Carbon atoms come in a variety of lengths.
106-112 pm for C-H single bond
120-154 p.m. – C-C single bond
205 p.m. for the C-Te single bond
The pattern is similar to the one observed for the atomic radius. Bond distances increase when a group of the periodic table is descended and decrease as a row or period is descended.
Distance Between Two Bonding Atoms
The bond length, or distance between two atoms that form a bond, can be determined experimentally. The X-ray diffraction of molecular crystals enables the three-dimensional structure of molecules to be determined and the internuclear distances to be precisely measured. There are also other spectroscopic methods for determining the bond length between two atoms in a molecule.
Relation Between Bond Length and Atomic Radii Of The Participating Molecule
The lengths of bonds between atoms are precisely proportional to the atomic radii of the atoms that are involved. Observable periodic patterns in the bond lengths of elements are similar to the periodic trends in the atomic radii of elements, which can be noticed in periodic trends in the bond lengths of elements (decreases across the period, increases down the group).
Periodic Trends in Bond Length
The atomic radii of the atoms involved in a bond determine the length of the bond. For example, periodic trends in the bond lengths and atomic radii of elements can be seen (decreases across the period, increases down the group).
The Bond with Shortest Bond Length
The actual distance between two atoms in a molecule is determined by factors such as orbital hybridization and the electrical nature of the molecule’s constituents, among others. Unlike other elements, hydrogen can form extremely short bonds; the shortest of them is H–H, which is only 74 pm in length.
Bond Lengths in Organic Compounds
Molecular bond length is determined not only by the atoms involved but also by other factors such as orbital hybridization and the electronic and steric character of the substituents used to form the bond. Diamond has a carbon–carbon (C–C) bond length of 154 pm, which is the longest known. Generally speaking, it is believed to be the average length of a carbon–carbon single bond, but it is also the longest bond length that can be found for conventional carbon covalent bonds. Because one atomic unit of length (i.e., a Bohr radius) is 52.9177 pm, the length of the C–C bond is 2.91 atomic units, or approximately three Bohr radii. The length of the C–C bond is 2.91 atomic units or approximately three Bohr radii.
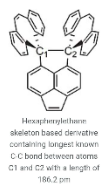
It is possible to have bond lengths that are unusually long. Currently, the 1,8-Bis(5-hydroxy dibenzo[a,d]cycloheptatrien-5-yl)naphthalene molecule holds the record for the longest C-C bond with a length of 186.2 pm. This molecule is one of many molecules belonging to the category of hexaaryl ethanes, which are derivatives based on the hexaphenylethane skeleton. The bond is positioned between the carbons C1 and C2, as shown in the illustration below.
Bond Length and Bond Order
The length of a bond is connected to the sequence in which it is formed when more electrons engage in the production of a bond, the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy if all other conditions are equal, a stronger bond will be shorter than one with a lower dissociation energy. A bond formed between two similar atoms has a covalent radius equal to half the bond distance between the two atoms.
Bond lengths are measured in the solid phase using X-ray diffraction, and in the gas phase, they are approximated using microwave spectroscopy, respectively. The strength of a bond formed between a given pair of atoms can differ between various molecules. In contrast to methyl chloride, the carbon-hydrogen bonds in methane are distinct from those in methyl chloride. When the general structure remains the same, however, it is easy to establish broad generalisations.
Conclusion
The bond length in chemistry refers to the equilibrium distance between the nuclei of two linked groups or atoms.The length of a bond is connected to the sequence in which it is formed when more electrons engage in the production of a bond, the bond is shorter.Bond lengths are measured in the solid phase using X-ray diffraction, and in the gas phase, they are approximated using microwave spectroscopy, respectively.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




