In the field of stereochemistry, the word Epimer refers to one of two stereoisomers. According to the stereogenic centre, the two isomers present in the molecules differ from one another while the remaining is similar. Some of the forms of glucose epimers include starch, glycogen, glucose, polysaccharides, and oligosaccharides.
The stereoisomers -D-mannopyranose and -D-glucopyranose are known as epimers since their sole difference in stereochemistry is in the C-2 position. The hydroxyl group in the -D-glucopyranose molecule is equatorial (in the “plane” of the ring), but the C-2 hydroxyl group in the -D-mannopyranose molecule is axial (up from the “plane” of the ring). These two molecules are known as epimers, however, they are not enantiomers since they do not mirror reflections of each other.
What are Epimers?
Epimers are a form of stereoisomer that has numerous stereocenters but differs only in the arrangement of one of the stereogenic centres. A stereocenter, also known as a stereogenic centre, is a carbon atom that is bonded to four separate atoms or groups of atoms. Consider the chemical ephedrine, which is used to treat asthma and to treat low blood pressure.
Take note of how the carbon atom is identified, one is connected to hydrogen, an alcohol group, a benzene ring, and another carbon atom. Likewise, the carbon atom labelled two is connected to hydrogen, nitrogen, a methyl group, and another carbon atom. Because they have four distinct groups linked to them, the atoms labelled one and two are both stereocenters.
Example
D-glucose and D-galactose are the best examples. Because both monosaccharides are D-sugars, the -OH group on carbon-5 of both hexoses is on the right in Fischer Projection. The sole difference between D-glucose and D-galactose is the carbon-4 position. In Fischer Projection, the -OH group for D-glucose is on the right, while the -OH group for D-galactose is on the left. This single difference results in the formation of D-glucose and D-galactose epimers. They are simply epimers, not enantiomers, diastereomers, or isomers.
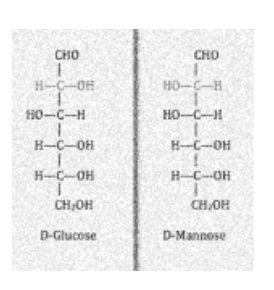
Epimerisation
Epimerization is the process by which various epimeric pairings produce epimers to demonstrate the process of stereochemistry at one of the positions of the carbon atom.
Stereoisomers
They are compounds that have the same chemical formula but distinct potential spatial configurations. The three-dimensional arrangement generated in the same is referred to as a special arrangement. Diastereomers and enantiomers are subsets of stereoisomers.
Enantiomers
Enantiomers are essential ‘arrangements of isomers in such a way that they are optically distinct from one another.’ In this case, the isomers created are mirror pictures of one other, which means they produce the same compound with the same formula and bonding between the individual atoms but have opposing orientations as in mirror images.
It should be emphasised that mirror images are never superimposable. These are known as optical isomers.
Diastereomers
It is commonly known as diastereoisomers, are a form of stereoisomer. They are known as non-mirror image non-identical stereoisomers. As a result, they are formed when two or more compound stereoisomers have distinct configurations at one or more (but not all) of the equivalent or related stereocenters and are not mirror reflections of one another. When two diastereomers vary from one other at just one stereocenter, they are referred to as epimers.
Anomers
Anomers are stereoisomers that form as a result of a variation in configuration at their anomeric carbon. Anomeric carbon is a carbon atom with an aldehyde or ketone group in an acyclic sugar molecule. The acyclic version of the sugar molecule is made up of an aldehyde or ketone group at one end and an alcohol group at the other. These two ends’ groups can react with each other to produce a cyclic sugar molecule to become more stable. The anomeric carbon has a –OH group connected to it in this cyclic configuration. The –OH group in one anomeric molecule is oriented in the opposite direction as the –OH group in the other molecule.
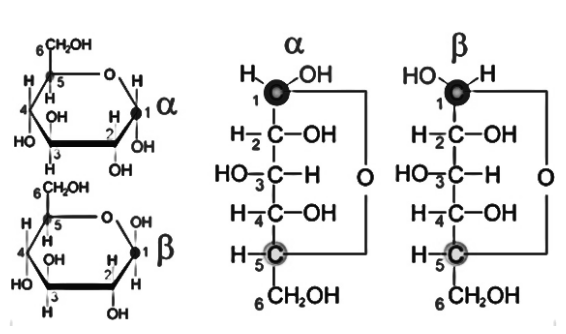
Anomerisation
Anomerization is the process of converting one anomeric form to the other. This is a procedure that can be reversed. Both anomers, however, are stable molecules having a cyclic structure. The two anomers are referred to as alpha (α) anomers or beta (β) anomers. The –OH group connected to the anomeric carbon of the alpha anomer is oriented in the opposite direction to that of the beta anomer of glucose, as shown in the above figure.
Difference Between Anomers and Epimers
S.NO | Anomers | Epimers |
1 | Anomers are stereoisomers that form as a result of a variation in configuration at their anomeric carbon. | Epimers are stereoisomers that vary from one another only at one chiral carbon. |
2 | Isomerism occurs in the anomeric carbons. | The epimeric carbon of the epimers undergoes isomerism. |
3 | Cyclic molecules are anomers. | Acyclic or cyclic compounds can be epimers. |
Conclusion
Epimers are a form of stereoisomer that has numerous stereocenters but differs only in the arrangement of one of the stereogenic centres. According to the stereogenic centre, the two isomers present in the molecules differ from one another while the remaining is similar. The stereoisomers -D-mannopyranose and -D-glucopyranose are known as epimers since their sole difference in stereochemistry is in the C-2 position. Epimers are a form of stereoisomer that has numerous stereocenters but differs only in the arrangement of one of the stereogenic centres. Epimerization is the process by which various epimeric pairings produce epimers to demonstrate the process of stereochemistry at one of the positions of the carbon atom.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



