Enantiomer, also known as enantiomorph, is one of two objects that are related to each other in the same way that the right hand is related to the left—that is, as mirror images that cannot be reoriented to appear identical. Because the object and its mirror image are identical, an object with a plane of symmetry cannot be an enantiomer. Molecular enantiomers, such as those of lactic acid, are chemically identical save in their reactions with other dissymmetric molecules and polarised light. Because many crystals are arrangements of alternate right- and left-handed versions of a single molecule, enantiomers are essential in
crystallography.
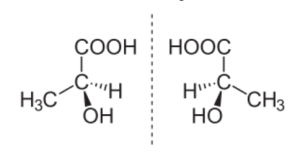
(S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are non-superposable mirror images of one another.
Enantiomers
The enantiomers have identical chemical and physical properties when they are present in a symmetric environment, except for their ability to rotate plane-polarized light (+/) by equal amounts but in opposite directions when they are present in an asymmetric environment (although the polarised light can be considered an asymmetric medium). Thus, such compounds are referred to as optically active, with specific terminology for each enantiomer based on the direction in which light is rotated: a dextrorotatory molecule rotates light in a clockwise (+) direction, whereas a levorotatory compound rotates light in the opposite direction (–). A racemic mixture, also known as a racemate, is a mixture that has an equal number of both enantiomers. If there is an equivalent amount of positive rotation in a racemic mixture, the amount of positive rotation is exactly counteracted by the same amount of negative rotation, and the net rotation is zero.
Enantiomer members frequently undergo chemical reactions with other enantiomer compounds that are opposed to one another. Because many biological compounds contain dimorphic enantiomers, there can be a significant difference between the impact of two dimorphic enantiomers on biological organisms. If medicine has two enantiomers, one of these is usually responsible for the desired physiological effects (referred to as the eutomer), while the other is less active, inactive, or sometimes even responsible for the undesirable physiological effects (referred to as the antitumour) (referred to as distomer). As a result of this discovery, medications made of only one enantiomer (“enantiopure”) can be designed to improve the efficacy of the drug while also reducing or eliminating some of its negative effects. For example, eszopiclone (Lunesta) is a single enantiomer of zopiclone, which is an older racemic medication with a similar name but different effects. This is an example of a chiral switch in its most basic form. Because only one of the two enantiomers appears to be responsible for all of the desirable effects, and the other enantiomer appears to be inactive, the dose of eszopiclone is exactly half of the zopiclone.
Chirality Centre
If a molecule does not have reflection symmetry or faulty rotation (rotoreflection) symmetry, it is chiral and, as a result, has an enantiomer; otherwise, it is not chiral. A typical feature of chirality is the existence of an asymmetric carbon atom with bonds to four separate atoms or groups, allowing these groups to be organised in two distinct non-superposable configurations. Chiral carbon atoms occur naturally in a wide variety of substances, including organic ones. Possessing an enantiomer because of an asymmetric carbon atom (or any asymmetrically-bonded atom of any element) is a common sort of chirality, which is referred to as point chirality or centre chirality in some circles. A chirality centre, which is a sort of stereocenter, is the name given to the asymmetric atom. Asymmetric atoms are always present in chiral compounds, whether they comprise exactly one (or any other odd number) of them. Meso compounds, on the other hand, are compounds that have an even number of asymmetric atoms but do not have chirality because they are grouped in mirror-symmetric pairs. Meso compounds are compounds that contain an even number of asymmetric atoms but do not have chirality. For example, meso tartaric acid (shown on the right) contains two asymmetric carbon atoms, yet it does not exhibit enantiomerism due to the presence of a mirror symmetry plane in the crystal structure. Asymmetric atoms are not required for all forms of chirality, such as axial, planar, and helical chirality, which are examples of chirality that does not require asymmetric atoms.
Properties Of Enantiomers
- Physical parameters such as melting temperature, boiling point, infrared absorptions, and nuclear magnetic resonance spectra are generally equal amongst enantiomers.
- The fact that, while the melting point and other properties of one enantiomer will be equal to those of the other enantiomer, the melting point of a mixture of the two enantiomers may be different is crucial to remember. This is because the intermolecular interactions between opposed enantiomers that are between the R and S enantiomers may be -different from those between similar enantiomers that are between two molecules that are both of R stereochemistry or both of S stereochemistry, respectively.
- The only class of physical techniques that can distinguish between the two enantiomers of a compound are chiroptical techniques, the most common of which is optical rotation. Chiroptical techniques are the only techniques that can distinguish between the two enantiomers of a compound.
- It is not only the lengths and angles of the bonds that determine the chiroptical properties of a molecule; it is also the sign and magnitude of the torsional angles that determine these properties, with the sign of the torsional angles being the only difference between enantiomers.
Conclusion
Enantiomers are in every other sense chemically similar. A pair of enantiomers is characterized by the direction in which when dissolved in solution they spin polarized light, either Dextro (d or +) or Levo (l or -) rotatory; hence the term optical isomers. When two enantiomers are present in identical proportions they are collectively referred to as a racemic mixture, a mixture that does not spin polarized light because the optical activity of one enantiomer is cancelled by the other.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




