Sulphur trioxide, or SO₃, is a chemical compound with the chemical formula SO₃. Sulphur trioxide comes in a variety of forms, each with its own molecular species and crystalline structure. It is colourless in liquid form and emits fumes in ambient settings. It is a highly reactive chemical that is also a strong oxidizer and a fire danger. In comparison to selenium dioxide, it is a thermodynamically unstable molecule. Sulfuric anhydride is one of the alternative names for sulphur trioxide.
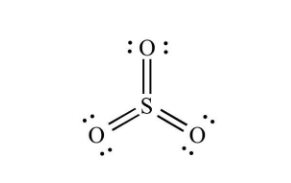
Structure of sulphur trioxide:
Properties of sulphur trioxide:
Physical properties of sulphur trioxide:
- Appearance: White crystalline solid; colourless.
- Odour: No odour.
- Complexity: 61.8
- Covalently-bonded unit: 1
- Solubility: Soluble in water.
- Hydrogen bond acceptor: 3
Chemical properties of sulphur trioxide:
When sulphur trioxide combines with water, sulfuric acid is formed. The chemical formula can be found below.
SO₃ + H₂O → H₂SO₄
When sulphur trioxide interacts with sodium hydroxide as a base, sodium hydrogen phosphate is formed. The chemical formula can be found below.
SO₃ + NaOH → NaHSO₄
Molecular structure and bonding:
- SO₃ is a D₃h symmetry trigonal planar molecule in its gaseous state, as predicted by VSEPR theory. As a result, SO₃ is classified as a member of the D₃h point group.
- The sulphur atom has an oxidation state of +6 and a formal charge of 0 according to electron-counting formalism. Without using the d-orbitals, the Lewis structure holds one S=O double bond and two S–O dative bonds.
- The electrical dipole moment of gaseous sulphur trioxide is 0. This is due to the 120-degree angle between the S-O bonds.
Preparation:
Let’s take a look at how to make sulphur trioxide using several methods:
In the atmosphere:
In air, sulphur dioxide is directly oxidised to sulphur trioxide, and this reaction is as follows:
SO₂ + 1/2O₂ → SO₃, ∆H = -198.4
The response described above occurs, although it is quite slow.
In the laboratory:
The sodium bisulfate compound is pyrolyzed in two stages in a chemical laboratory to produce sulphur trioxide. As an intermediate product, sodium pyrosulfate is given:
The chemical reaction for dehydration at 315 °C is:
2 NaHSO₂ → Na₂S₂O₇ + H₂O
The reaction can be written as:
Na₂S₂O₇ → Na₂SO₄ + SO₃
KHSO4 molecules, on the other hand, do not undergo the same reaction.
It’s also possible to make it by mixing concentrated sulfuric acid with phosphorus pentoxide.
In industry:
The contact process can be used to produce SO₃ in the industrial setting. Sulphur dioxide is created by the combustion of iron pyrite (a sulphide mineral of iron) or sulphur. After being purified by electrostatic precipitation, the SO₂ compound is oxidised by air oxygen over a catalyst at a temperature of 400 to 600 °C. On a silica or kieselguhr substrate, a common catalyst contains vanadium pentoxide (V₂O₅) activated with potassium oxide K₂O. Platinum is also very effective, but it is much more expensive and is poisoned (or rendered ineffective) by impurities much more easily.
Uses of sulphur trioxide:
- This can be used as a bleaching agent to eliminate any remaining hydrogen peroxide or a digesting agent to separate the pulp from the lignin.
- It can be used as a catalyst in the oxidation of sulphur dioxide to sulphur trioxide.
- Strong inorganic acid mists containing sulfuric acid are utilised either in industrial or in commercial product manufacture.
- In sulfonation processes, it is also a significant reagent.
- It’s a material that can be utilised to make solar energy gadgets and photoelectric cells.
Safety:
Because it is highly hygroscopic and corrosive, sulphur trioxide, in addition to being a strong oxidising agent, will cause serious burns when consumed or inhaled. Because SO3 interacts violently with water and creates highly corrosive sulfuric acid, it should be handled with extreme caution. It should also be kept away from organic materials due to its severe dehydrating properties and its potential to react strongly with them.
Conclusion:
In the sulfonation processes, sulphur trioxide is a necessary reagent. Dye, medicines, and detergents are all made possible by these procedures. Sulphur trioxide can be produced in situ from sulfuric acid, or it can be added to the acid as a solution.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





