In physics, we come across a variety of quantities with different properties, and characterising them gets problematic when we talk about terms like energy, time, work, or any other physical quantity that requires a standard measure to identify. The units establish the standard measure of certain quantities in physics. For example, let’s imagine a person weighs 36 kilograms and lives 1200 kilometres away from a city. The units used to define these physical quantities are kg (for weight) and km (for distance). Kelvin is another example of a temperature unit; and so on.
Because energy is measured in terms of work, the joule (J) is the SI unit of energy. It was named after James Prescott Joule and his research into the mechanical equivalent of heat.
In simpler terms, one joule equals one newton metre and, in terms of SI base units,

The energy unit is the same as the work unit (As work and energy are the two sides of the same coin).
Energy
We’ve been hearing a lot about energy lately. Every invention, every civilization, is built on the acquisition and efficient use of energy. This is made possible by our Universe’s unique ability to transfer and transform energy while maintaining the same overall amount (conserved).
The study of energy is one of the most important aspects of physics. Energy is a scalar quantity in physics that is related with the state or condition of one or more objects.
Types of Energy
Kinetic energy: It has something to do with an object’s state of motion. The more kinetic energy it has, the faster it moves.
Mathematically, Kinetic Energy is expressed as:
K=1 ⁄ 2mv²
Here,
K= Kinetic Energy
m= mass of the body
v= velocity of the body
Potential energy: It has to do with the arrangement (configuration) of a system of items that exert forces on one another.
U=mgh
Here,
U= potential energy
m= mass of the body
g= acceleration due to gravity
h= height of the body above ground
Work and Potential energy
If the ball is thrown up in the example above, the work done by the gravitational force is negative. The ball’s velocity (kinetic energy) declines as it climbs, eventually reaching zero when it reaches its maximum height. We can therefore deduce that the energy contained in the ball at its highest height is gravitational potential energy.
The potential energy is used to store the work done by the gravitational force. As a result, the change in potential energy is defined as the negative of the work done on the system in every scenario.
∆U=-W=-F.∆x
Here,
F= constant force
∆x=the displacement
If the force is a conservative force, the change in potential energy is given by,
∆U=r1r²F(r)dr
The conservative force from potential energy can be calculated as follows:
F(r)=-dU⁄ dx=0
Potential Energy and Equilibrium
If the net force acting on is equal to zero, the object is said to be in equilibrium.
F(r)=-dU ⁄ dx=0
Stable equilibrium: If a body is in stable equilibrium, it will return to its original position after a minor movement. The restoring force should be applied to minor displacements.
Unstable equilibrium: If a body does not return to its normal position after a minor displacement, it is in unstable equilibrium. The restoring force should remove the object from a little displacement.
Neutral equilibrium: The particle can be moved significantly away from such a place and still remain in equilibrium (i.e., it will neither attempt to return to its initial state nor will it continue to move)
SI Unit of Energy
The S.I unit of Energy is Joule.
Interestingly, the name of this international unit is kept in honour of James Prescott Joule, a British physicist whose works contributed to the establishment of the energy concept. When we look at the unit in fundamental terms, 1-N.m is equal to 1 Joule.
CGS Unit of Energy
An erg is a unit of energy equal to 10-7J. The amount of effort done by a single dyne across a one-centimetre distance is measured in ergs.
Unit of Energy in MKS System
metre-kilogram-second is the abbreviation for MKS.
1 Joule equals one Newton-metre (N-m), where 1 J is the amount of work a newton of force does over a distance of one metre.
Here,
1J=kgm²/s²=1 Watt second
MKS unit of energy = kgm²/s²
Units Of Energy List
Different units are commonly used to measure energy, and energy units can be preceded by a variety of elements. The following are some examples of common units:
- British thermal unit (BTU)
- Horsepower
- Kilowatt-hour (kWh)
- Calorie
- Electron volts (eV)
- Hartree (the atomic unit of energy)
- Rydberg units
- Barrel of oil
Commercial Unit of Energy
The commercial unit of energy is the kilowatt-hour.
The SI unit of energy becomes little when huge amounts of energy need to be expressed. As a result, the commercial unit is used.
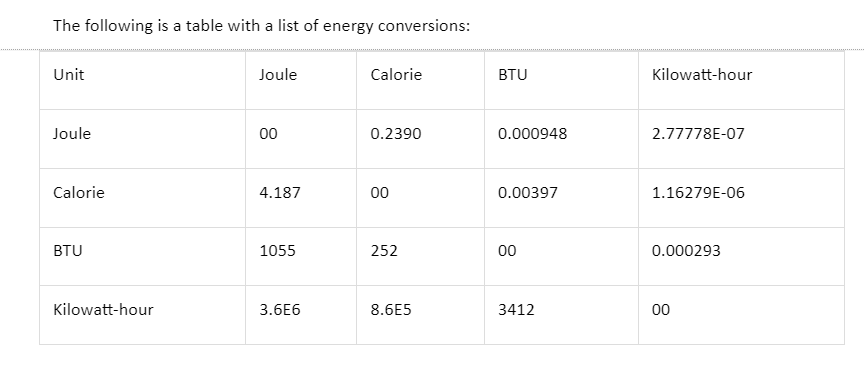
Energy Conversion
Conclusion
The unit of energy is the Joule, according to the International System of Units (SI) (J). In addition, energy is measured in a variety of different units that are not part of the SI system. Calories, ergs, kilowatt-hours, kilocalories, and British Thermal Units are examples of these units. If we want to represent this in SI units, we’ll require a conversion factor. All of these are employed in trade and science in some capacity.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



