Depending on the wave prototype of light, physicists assumed that enhancing light amplitude would increase the kinetic energy of discharged photoelectrons while enhancing the frequency would show an increment in the measured current.
On the contrary, experiments stated that enhancing the light frequency and expanding the light frequency causes the increment in the kinetic energy of the photoelectrons, and enhancing the light amplitude shows an increment in the current. Depending on these studies, Einstein stated that light acted like a stream of particles known as photons possessing energy of E=hv.
The photoelectric effect is a phenomenon where light shines on a metal surface.
In terms of the properties of photoelectrons, they are nothing different from other electrons. We have discussed much deeper aspects related to what is the photoelectric effect.
Definition And Description Of The Theory
The photoelectric effect is an incident where electrons are discharged from the surface of a metal when light falls onto the surface. The discharged electrons are considered photoelectrons. It is crucial to spot that the emission of photoelectrons and the kinetic energy of the discharged electrons depend on the frequency of the light that falls on the metal’s surface.
The process by which photoelectrons are discharged through the surface of the metal for the action of the incident light is specifically called photoemission.
The photoelectric effect takes place as the electrons present on the surface of the metal are inclined towards absorbing energy from the incident light and utilise it to conquer the attractive forces which connect them with the metallic nuclei.
The photoelectric effect does not speak about the wave property of light. However, this effect can be illustrated by the particle property of light, where light can be studied as a stream of particles of electromagnetic energy.
These particular particles of light are known as photons. The energy captured by a photon is associated with the frequency of light through the Planck’s constant.
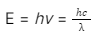
Here,
- E = energy of the photon
- h = Planck’s constant
- 𝜈 = the frequency of the light
- c = speed of light (in a vacuum)
- λ = wavelength of the light
Hence, it can be said that various frequencies of light transfer photons of different energies. For example, the frequency of blue light is much larger than that of red light. So the energy captured by a photon of blue light will be bigger than the energy captured by a photon of red light.
Discovery of The Photoelectric Effect
German physicist Heinrich Rudolf Hertz discovered what is the photoelectric effect in 1887. When he studied radio waves, he saw that when UV light illuminates on two metal electrodes possessing a voltage given across them, the light alters the voltage due to which sparking happens. This connection between light and electricity was explained by Philipp Lenard later on. He said that electrically charged entities are discharged from a metal surface when it is illuminated. These particles are similar to electrons, established by a British physicist named Joseph John Thompson in 1897.
Further studies showed that the Einstein photoelectric effect shows a connection between light and matter that is incapable of being explained by the properties of classical physics, which says light possesses electromagnetic waves. One complicated observation was that the larger kinetic energy of the released electrons was not varying with the severity of the light, as expected depending upon the wave theory, but was proportional instead to the intensity to the frequency of the light.
Light intensity could not determine the amounts of electrons discharged from the metal. Another complicated observation was there was actually no time difference between the appearance of radiation and the emission of the electrons.
Examinations of this complicated and unusual information led Albert Einstein to examine a new corpuscular theory of light where every particle of light or photon possesses a specific amount of energy, or quantum, which relies on the light’s frequency.
In particular, a photon possesses an energy E equivalent to hf, where f denotes the frequency and h denotes the universal constant derived by Max Planck. The connection might also be stated in the wavelength, depicting that the energy of a photon is inversely proportional to its wavelength.
Einstein thought that a photon would be able to penetrate the material and convey its energy to an electron. Because the electron shifted via a metal with excessive speed and ultimately came up from the material, its kinetic energy would eventually diminish by an amount ϕ known as the work function, which shows the energy needed for the electron to move from the metal. By conservation of energy, this point of view led Einstein toward the photoelectric equation Ek = hf − ϕ, where Ek is the highest kinetic energy of the discharged electron.
Conclusion
Summarising the whole topic, we can say that depending on the wave model of light; physicists said that enhancing light amplitude would enhance the kinetic energy of the discharged photoelectrons while enhancing the frequency is going to increase the measured current.
Experiments stated that enhancing the light frequency caused the kinetic energy of photoelectrons to increase, and enhancing the light amplitude also increased the current.
Depending on these findings, Einstein stated that light behaved like a stream of photos possessing energy of E=hv.
Knowing what the photoelectric effect is important in developing knowledge about quantum physics and solving sums related to it will develop your insights even more.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




