Isothermal reactions can take place in any system that has a temperature control mechanism, including highly organised machines and even living cells. When looking at the thermodynamics of chemical reactions, it’s common to look at what happens under isothermal conditions first, then the influence of temperature. Phase changes, such as melting or evaporation, are also isothermal processes when they occur at constant pressure, as they normally do. Isothermal processes are frequently used as a starting point for evaluating non-isothermal processes that are more complex.
T=constant
T=0
dT=0
U=0 (for ideal gases only)
Q=0 (for adiabatic process)
Isothermal process work equation
The reversible work involved when a gas transitions from state A to state B in thermodynamics is:
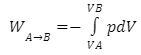
where p denotes gas pressure and V denotes gas volume.This integral equals the area under the relevant PV (pressure-volume) isotherm for an isothermal (constant temperature T), reversible process. Again, p = nrt/V applies, and since T is constant (due to the fact that this is an isothermal process), the work equation becomes:
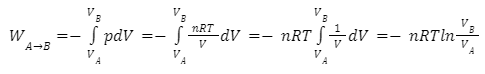
Work is defined by the IUPAC convention as work performed on a system by its surroundings. If the system is compressed, for example, the surrounding performs work on it, resulting in positive work and an increase in the system’s internal energy.It’s also worth mentioning that, for perfect gases, if the temperature is kept constant, the system’s internal energy U is similarly constant, hence ΔU = 0. In IUPAC convention, the First Law of Thermodynamics states that Δ U = Q + W, hence Q = –W for isothermal compression or expansion of ideal gases.
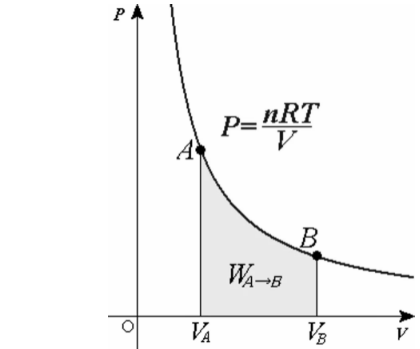
What is isotherm?
If we observe the relationship between temperature and any other thermodynamic variable such as pressure, volume, and others and draw a graph on the Cartesian plane, we can call all the curves that represent two states of a system where the temperature is the same during an isothermal process isotherms.In an isothermal process, an isotherm is a series of curves or lines that represent two states of a system at the same temperature.
During an isothermal process, for example, a line drawn as shown in the image below has two states A and B, both of which are at the same temperature, so the line is termed isotherm. We can look at any new thermodynamic variable on the X-axis.
Isothermal process Examples
Many examples of isothermal processes can be found in our everyday lives. The isothermal process can be regarded as reversible because the temperature remains constant and the only changes are in the volume and pressure of the components. Here are a few examples:
- Melting or evaporation causes a change in the phase or state (solid, liquid, or gas) of things.
- At a steady temperature, the Carnot Cycle operates.
- Isothermal processes are those that take place in the refrigerator and take place at a constant temperature.
- Interactions between one cell and its surroundings occur in our body as a result of the isothermal process.
- Through the Isothermal process, the heat pump either transfers heat into the house or shifts heat outside of the house.
Properties of isothermal process
- dT = 0 is the condition for an isothermal process.
- The formula PV = nRT can be used to calculate Boyle’s Law for an ideal gas in an isothermal process.
- The general formula for the internal energy of an ideal gas is U = 3/2 nRT, which is derived from the first rule of thermodynamics.
- The system’s temperature remains constant.
- PV=constant is the state equation.
- W = nRTln(V2/V1) is the work done by n moles of an ideal gas in an isothermal expansion from volume V1 to V2 at temperature T.
- The internal energy change, i.e. U, is zero.
- The amount of heat provided to the gas is proportional to the amount of work done by the gas.
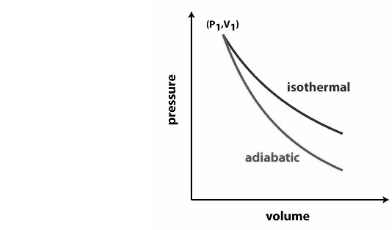
Isothermal process vs adiabatic process
A thermodynamic process known as an adiabatic process occurs when there is no heat transfer between the system and its surroundings. It does not specify, however, that the temperature is not consistent during the operation. It simply means that no heat is being transported into or out of the system. It’s a process that can be reversed.
A thermodynamic process in which the temperature of the system remains constant is known as an isothermal process. It implies that even if the system’s pressure or volume changes, the temperature remains constant. There is a heat transfer in the system, but it is too slow to maintain thermal equilibrium.
Conclusion
When scientists investigate isothermal processes in systems, they are primarily interested in heat and energy, and how they relate to the mechanical energy required to change or maintain the temperature of a system. This knowledge assists biologists in their investigation of how living things regulate their temperatures. It is also used in engineering, space science, planetary science, geology, and other fields. The concept of thermodynamic power cycles underpins heat engines (and thus isothermal processes). As previously stated, humans use these devices to power electrical generating plants, as well as cars, trucks, planes, and other vehicles. These systems are also found on rockets and spacecraft. Engineers use thermal management techniques to improve the efficiency of these systems and processes. One of the important thermodynamic processes, isothermal processes, is discussed in this article along with its applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




