- The photoelectric effect occurs when a material absorbs electromagnetic radiation and emits electrically charged particles from or within it. The phenomena of a substance (metal) emitting electrons when subjected to suitable frequencies of radiation is known as the photoelectric effect, and the produced electrons are known as photoelectrons.
Photons
A photon, often known as a quantum, is the smallest distinct amount of electromagnetic energy. It is the basic unit of all light.
In a vacuum, photons are always in motion and travel at a constant speed of 2.998 x 108 m/s to all observers. The letter c stands for the speed of light. Each photon has its own amount of energy and momentum. The photon’s energy is given by the relation,
E=hν
Here, E denotes the energy of the photo, h denotes the planck’s constant and denotes the frequency of the photon.
A photon’s momentum is given by,
p=h
Here, p is the momentum of the photon, h is the planck’s constant and is the wavelength of light
Properties of photon
- As light intensity rises, so does the number of photons crossing an area per unit time. It has no influence on the energy of the radiation.
- A photon is unaffected by electric or magnetic fields. It is devoid of any electrical charge.
- A photon has no mass.
- It’s a very well-built particle.
- Photons can be created or destroyed when radiation is emitted or absorbed.
- The total energy and momentum of a photon-electron collision is conserved.
- A photon cannot decompose on its own.
- When a photon interacts with other particles, its energy can be transmitted.
- A photon has a spin of one, unlike electrons, which have a spin of 1/2. Its spin axis runs perpendicular to the direction of flight. This property of photons aids in the polarization of light.
Photoelectric effect
When incoming light with an energy larger than the metal’s threshold value strikes the surface, the metal’s tightly bound electrons are released. A picture is a tiny speck of light. When a photon collides with an electron, the sum of its energy is transferred to the electron, causing the electron to eject off the surface. The photon’s remaining energy is converted into a free negative charge known as a photoelectron.
Einstein’s explanation
- The photoelectric current’s strength is determined by the intensity of incident radiation and should be greater than the threshold frequency.
- The photo-current stop was the reverse stopping possibility. It is unaffected by incident radiation intensity.
- If the frequency of the incident radiation is less than the threshold frequency, photoelectric current does not exist. When exposed to light or the sun, a metallic strip will not be able to produce the Photoelectric effect unless the frequency exceeds the threshold value.
- The photoelectric effect occurs in a split second. The metal’s electrons are ejected as soon as light strikes the surface.
Conditions required for photoelectric effect
- Threshold Frequency (Yth): The metal’s threshold frequency is the lowest frequency of incident light or radiation that causes a photoelectric effect, i.e., photoelectrons to be ejected from a metal surface. For one metal, it is constant, but different metals may have different values.
If Y= incoming photon frequency and Yth threshold frequency, then
- If Y<Yth, no photoemission occurs, as a result, no photoelectric effect will occur.
- If Y=Yth , photoelectrons are simply ejected from the metal surface, and the kinetic energy of the electron is zero.
- If Y>Yth, the surface will be ejected of photoelectrons and kinetic energy.
- Threshold Wavelength (th): During electron emission, the metal surface with the greatest wavelength to incident light is known as the threshold wavelength.
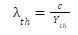
If the incident photon’s wavelength is , then
- If λ<th occurs, the photoelectric effect occurs, and the expelled electron has kinetic energy.
- Ifλ=th , only the photoelectric effect will occur, and the kinetic energy of the emitted photoelectron will be zero.
- If λ>th, no photoelectric effect will take place.
Work function or threshold energy : The minimal amount of thermodynamic work necessary to remove an electron from a conductor to a place in the vacuum just outside the conductor’s surface is known as the work function/threshold energy.
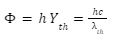
If E is the energy of a photon that has been incident, then
- Photoelectric effect will not take place if E<.
- If E=0, just the photoemission occurs, but the photoelectron’s kinetic energy is 0.
- If E> then the photoelectric effect will occur, as will the kinetic energy of the expelled electron.
Conclusion
The electron-bound electron is released when high-energy light passes through a threshold and impacts a metal surface. When a photon collides with an electron, it releases the electron from the metal with some of its energy. The photon’s remaining energy is transmitted to the photoelectron, which is a negative charge. The photoelectric effect is what causes this.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




