Introduction:Meaning
Perchloric acid is a naturally occurring mineral acid. This colorless chemical, which is usually found as an aqueous solution, is a stronger acid than sulfuric acid, nitric acid, and hydrochloric acid. When heated, it is a potent oxidant, however at ambient temperature, aqueous solutions up to about 70% by weight are normally harmless, with only strong acid characteristics and no oxidizing properties.
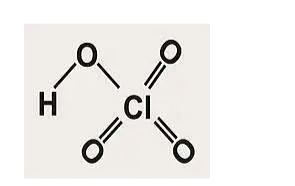
Perchloric acid formula and structure:
The formula for perchloric acid is HCLO4.
The structure is as follows:
Perchloric acid preparation:
- Perchloric acid precipitates solid sodium chloride when a high aqueous solution of sodium perchlorate (209 g/100 mL of water at room temperature) is treated with hydrochloric acid:
NaClO4 + HCl → NaCl + HClO4
- Perchloric acid precipitated barium sulfate when barium perchlorate is treated with sulfuric acid.
Ba (ClO4)2 + H2SO4 → BaSO4 + 2HClO3
- Perchloric acid is made by further evaporating the HClO3 solution.
3HClO3 → HClO4 + Cl2 + 2O2 + H2O
- Adding hydrochloric acid to a solution of nitric acid (HNO3) and ammonium perchlorate (NH4ClO4) while the mixture is boiling.
Properties:
IUPAC Name | Perchloric acid |
Physical appearance | Clear, colorless, and odorless aqueous solution and corrosive to metals and tissues |
Chemical Formula | HClO4 |
Density of Perchloric acid | 1.768 g/cm3 |
Molecular weight of HClO4 | 100.46 g/mol |
Melting point of Perchloric acid | −17 °C |
Boiling point of Perchloric acid | 203 °C |
Aqueous pKa | −15.2±2 |
Perchloric acid uses:
Perchloric acid has the following applications:
- It is utilized as a precursor to ammonium perchlorate, a critical component of rocket fuel.
- It’s used to separate sodium and potassium as an oxidizer.
- In the space industry, it is an important compound.
- It’s used in the production of explosives.
- It’s used in Liquid Crystal Display (LCD) etching techniques (LCD).
- It is used in metal plating.
- It’s used as a reagent to determine the 1H-Benzotriazole.
- It’s used to electropolish or etch chrome and molybdenum.
- Perchloric acid is one of the main components of Bronsted-Lowry because of its extremely acidic characteristic.
- It is crucial in the extraction of materials from their ores.
- It is employed in analytical chemistry because of its unusual characteristics.
- It’s also used in the electronics sector.
Why is perchloric acid considered the most powerful acid on the planet?
Perchloric acid is corrosive in nature. In the structure, one of the oxygen atoms forms a single bond with chlorine, while the other three oxygen atoms are linked to chlorine via a coordinate bond.Acidic acids are H+ proton-rich acids, whereas strong acidic acids are those with persistent conjugate bases. The negative charge conjugation produced on the oxygen atom and the other three oxygen atoms makes perchlorate ions stable.
Safety:
Perchloric acid is heavily regulated due to its powerful oxidizing effects. It reacts violently with metals (such as aluminum) and biological materials (wood, plastics). To avoid the accumulation of oxidisers in the ductwork, work with perchloric acid must be done in fume hoods with a wash-down capability.
On February 20, 1947, a bath containing approximately 1000 liters of 75 percent perchloric acid and 25 percent acetic anhydride by volume burst in Los Angeles, California, killing 17 persons and injuring 150 more. The O’Connor Electro-Plating plant, along with 25 other buildings and 40 automobiles, was destroyed, and 250 nearby homes were damaged. In the bath, electro-polishing aluminum furniture was done. Organic compounds were added to the hot bath when an iron rack was replaced with one covered in cellulose aceto butyrate.The bath exploded a few minutes later.
Hazards of Perchloric acid:
Perchloric acid has the following health risks:
- This compound’s vapors create a burning feeling in the nose, throat, and lungs when inhaled.
- This substance causes vomiting when exposed to it for an extended period of time.
- This chemical can cause stomach blisters and burns if consumed.
- It is also extremely caustic to human skin.
It is very reactive to most metals due to its oxidizing nature, and when heated, it releases irritating, corrosive, and poisonous fumes. As a result, this substance should be handled with caution.
Conclusion:
Anhydrous perchloric acid is an oily, unstable liquid at room temperature. It produces at least five hydrates, some of which have been discovered crystallographically. In these materials, hydrogen bonds bind the perchlorate anion to the H2O and hydronium ion centres. Perchloric acid reacts with water to generate an azeotrope, which contains 72.5 percent perchloric acid. This acid is readily available in stores and has an infinite shelf life. Hygroscopic solutions are those that are hygroscopic. If exposed to the air, concentrated perchloric acid dilutes by absorbing water from the air.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




