Free radicals are highly reactive and unstable molecules that are produced in the body naturally as a by-product of normal metabolism, or by exposure to toxins in the environment such as tobacco smoke and ultraviolet light. Free radicals have a lifespan of only a fraction of a second, but during that time can damage DNA, sometimes resulting in mutations that can lead to various diseases, including heart disease and cancer. Antioxidants in the foods we eat can neutralize the unstable molecules, reducing the risk of damage. The free radicals are produced during ATP through mitochondria. They are generally divided into two well-known entities: reactive oxygen species and reactive nitrogen species
What is free radical:
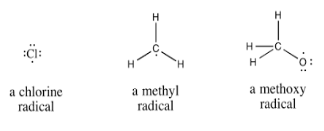
Chemical species having one or more unpaired electrons are called free radicals. Homolytic bond fission leads to the formation of free radicals. The free radicals are odd electron molecules and are highly reactive. Free radicals are paramagnetic in that they possess a small permanent magnetic moment due to the presence of unpaired electrons. This property is used for the detection of the presence of free radicals.
A notable example of a radical is the hydroxyl radical, a molecule that has one unpaired electron on the oxygen atom. The two other examples are triplet oxygen and triplet carbene (:C H2) which have two unpaired electrons.
Types of free radicals:
Most organic radicals are quite unstable and very reactive. There are two kinds of radicals, neutral radicals, and charged radicals.
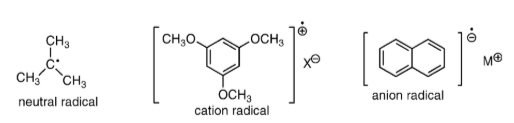
Neutral radical: These free radicals do not charge them that’s why they have a much lesser reactivity than the charged free radicals. Atoms have unpaired electrons if they have an odd number of electrons. It can be electrically neutral because there are as many as protons, so the net charge is zero.
- Cation radical: A radical cation is a positively charged species that has an atom bearing an unpaired electron.
For example, some radical cations are resonance stabilized. Radical cations are common intermediates in reactions occurring in a mass spectrometer.
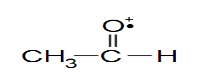
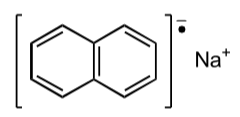
Anion radical: Radical anion is a subset of charged free radical species that carry a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds. For example, sodium naphthene.
Moreover, there are two types of radicals: the sigma radicals and the pi radicals. An unpaired electron in the sigma-radical is in the sigma orbital and an unpaired electron in the pi radical is in the pi orbital respectively. Therefore, the radicals above are pi radicals, t-Butyl radical is also a pi radical since this radical is stabilized by hyperconjugation. However, the phenyl radical and the vinyl radical are typical sigma radicals.
Normally, pi radicals are stabilized by the hyperconjugation effect or the resonance effect. However, sigma radicals are very reactive because there is no such stabilizing effect.
Examples of free radicals:
Consider three reactive species methyl anion, methyl cation, and methyl radical. These radicals are shown below.
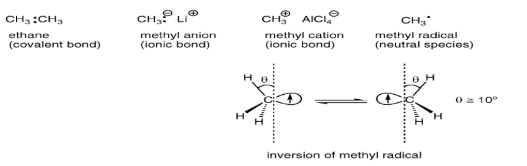
Ethane is composed of two methyl groups connected by a covalent bond and is a very stable compound. The methyl anion and methyl cation have an ionic bond mainly between carbons and counter ions respectively and are not particularly unstable though there are some rather moisture-sensitive species.
However, the methyl radical is an extremely unstable and reactive species, because its octet rule on the carbon is not filled. The carbon atom in the methyl cation adopts sp2 hybridization and the structure is triangular and planar. The carbon atom in the methyl anion adopts sp2 hybridization and the structure is tetrahedral, However, the carbon atom in the methyl radical adopts a middle structure between the methyl cation and the methyl anion, and its pyramidal inversion rapidly occurs even at extremely low temperatures.
Some other types of free radicals are:
The most important free radical which consists of oxygen are hydroxyl radical
- Superoxide anion radical
- Hydrogen peroxide
- Hypochlorite
- Nitric oxide radical
- Peroxyntrite radical
Superoxide oxygen: It is generated by direct auto-oxidation of O2 during mitochondrial electron transport reaction. Alternatively, O2. Is produced enzymatically by xanthine oxidase and cytochrome p450 in the mitochondria.
Nitric oxide radical: is a diatomic free radical that regulates biological functions. NO reacts and interacts readily with thiols and transition metals.
Peroxynitrite radical: peroxynitrite is an ion with the formula ONOO–. It is an unstable structural isomer of nitrate, NO-. it is an oxidant and nitrating agent. Because of its oxidizing properties, it can damage cells.
Hydroxyl radical: It is the most reactive free radical and can be formed from O2 and H2O2 in the presence of metal ions such as copper or iron. Hydroxyl radicals have the highest electron reduction potential and are primarily responsible for the cytotoxic effect in aerobic organisms.
Conclusion:
Chemical species having one or more unpaired electrons are called free radicals. Homolytic bond fission leads to the formation of free radicals. The free radicals are odd electron molecules and are highly reactive. Most organic radicals are quite unstable and very reactive. There are two kinds of radicals, neutral radicals, and charged radicals.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




