Borax is better known as a home detergent, but it’s also an important component in water treatment, according to Bell Chem, a water treatment chemical provider. Borax is also known as sodium borate, sodium tetraborate, disodium tetraborate, or sodium tetraborate decahydrate, and has the chemical formula Na2B4O710H2O. Borax and boric acid are both boron compounds, although they are not the same. Borax is a naturally occurring mineral that can be mined underground or collected from evaporation deposits above ground. Boric acid is the refined chemical that results from the processing of this mineral. In other words, borax is a boric acid mineral salt.
Chemical Reactivity of Borax with Water
Borax is a boron-containing mineral found in nature as a white powder made up of soft, soluble crystals. Silk-road pilgrims discovered borax in dry lake beds in Tibet, and it was later discovered in the California and Nevada deserts of America. It’s a key element in several soaps and detergents, as well as a soldering flux, an antifungal agent, and a texturizer in some prepared meals. It’s also used to make “Pyrex,” a thermoresistant borosilicate glass popularly known as “Pyrex.” You may be most familiar with borax through the name 20 Mule Team Borax, which is a laundry additive.
The empirical formula of the mineral borax in its natural state is Na2B4O710H2O, and its IUPAC name is sodium tetraborate decahydrate, but we’ll just call it “borax” here. Figure 1 depicts the structure of the tetraborate anion,B4O5OH42-. This image shows that the chemical formula Na2B4O5OH48H2O gives a more structurally correct description of the borax molecule, which is the one we’ll use here.
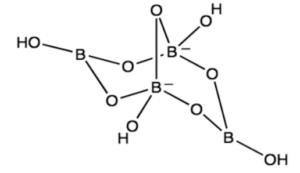
The following is the chemical equation for the dissolution of borax:
Na2B4O5OH48H2O ⇌ 2Na+aq+ B4O5OH42-aq+ 8H2O
The weak base tetraborate anion produced in solution reacts with water as follows:
B4O5OH42-aq+ 5H2Oaq⇌ 4H3BO3aq2+OH–aq
The concentration of tetraborate anion in solution (and consequently the amount of dissolved borax) can be calculated as follows: To begin, we take a sample of the saturated solution at equilibrium, being cautious to only take the liquid component and not any of the solid borax that is in equilibrium with it. The hydroxide ions are then titrated with a strong acid, such as HClaq. According to Le Chatelier’s principle, as the acid removes the hydroxide ions from solution, additional tetraborate anions are transformed into boric acid and hydroxide ions. That is, as the strong acid removes OH– ions from solution, the process given by Equation shifts to the right. During the titration, we can represent the overall reaction between the tetraborate anions and the hydronium ions formed from the strong acid, H3O+, as follows:
B4O5OH42-aq+2H3O+aqH2O ⇌ 4H3BO3aq
The reaction given by Equation will continue until the titration reaches equivalence. All of the tetraborate anions in the solution have now been transformed to boric acid, and all of the OH– ions have been neutralized. The addition of extra strong acid causes the pH of the solution to shift quickly from basic to acidic since all of the base has been neutralized. The presence of an acid-base indicator allows us to see this change and will serve as a marker for when our titration is finished. The indicator for this titration will be bromocresol green, which changes color from blue to yellow when acid is added at a pH of 4.8.
Characteristics of Borax
According to Science Lab, the chemical formula for borax, sodium borate, is B4O7∙2Na. Borax is an odorless solid that weighs 201.22 g/mole and melts at 741°C. In the presence of glass, borax is stable and non-corrosive. According to Science Lab, it is incompatible with alkaloidal salts, mercuric chloride, zinc sulfate, and other metallic salts. Moisture is also incompatible with it. Borax is also known as boric acid, disodium salt, disodium tetraborate decahydrate, sodium borate, sodium borate decahydrate, sodium tetraborate, sodium tetraborate decahydrate, sodium tetraborate decahydrate, sodium tetraborate decahydrate, sodium tetraborate decahydrate, sodium tetraborate decahydrate, sodium tetraborate decahydrate, White, gray, blue, or greenish-white streaking crystals with a water solubility of 6g to 100g The density of sodium borate is 1.73, and its boiling point is 320 degrees Celsius.
Borax and Water
Borax: Borax is a powdery white chemical that is also known as sodium borate, sodium tetraborate, or disodium tetraborate. It’s a typical household cleaner and laundry detergent booster. All three elements are present: boron, sodium, and oxygen.
Water: Water H2O is an inorganic, clear, tasteless, odorless, and almost colorless primary constituent of the Earth’s hydrosphere and all known living things’ fluids is a chemical compound called chlorophyll. It is required for all known forms of life, despite the fact that it contains no calories or organic nutrients.
Conclusion
Borax is commonly discovered in dry lake beds, such as those found in California’s Death Valley, where water evaporated and left behind mineral deposits.
Boric acid is made from the same chemical compound as borax and has a similar appearance to it. Borax is often used for cleaning, although boric acid is mostly used as a pesticide. Boric acid attacks insects’ stomachs and neurological systems, killing them.
If ingested, both borax and boric acid in loose powder form can be dangerous, especially to children. They can irritate your skin as well.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




