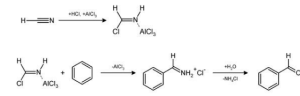
Gattermann formylation and Gattermann salicylaldehyde synthesis, the Gattermann reaction is a chemical reaction in which aromatic compounds are formulated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst, such as AlCl3. The reaction is named after the German chemist Ludwig Gattermann, and it is quite similar to the Friedel–Crafts reaction in its characteristics.
 It has been demonstrated through modifications that it is possible to substitute sodium cyanide or cyanogen bromide for hydrogen cyanide.
It has been demonstrated through modifications that it is possible to substitute sodium cyanide or cyanogen bromide for hydrogen cyanide.
Gattermann formylation
Named after | Ludwig Gattermann |
Reaction type | Substitution Reaction |
It is possible to simplify the reaction by using zinc cyanide for the HCN/AlCl3 combination in the first step. Even though it is a highly hazardous substance, Zn(CN)2 is a solid, making it safer to work with than HCN, which is a gas. The Zn(CN)2 combines with the HCl to create the important HCN reactant as well as Zn(Cl2), which acts as the Lewis-acid catalyst in the presence of the HCl. The synthesis of mesitaldehyde from mesitylene is a good example of the Zn(CN)2 technique in action.
Gattermann–Koch reaction
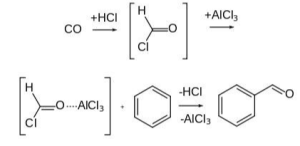
This reaction, named after the German scientists Ludwig Gattermann and Julius Arnold Koch, is a version of the Gattermann reaction in which carbon monoxide (CO) is employed instead of hydrogen cyanide (HCN). It is named after the German chemists Ludwig Gattermann and Julius Arnold Koch.
Gattermann–Koch formylation
Named after | Ludwig Gattermann Julius Arnold Koch |
Reaction type | Subsititution Reaction |
It should be noted that, in contrast to the Gattermann reaction, this reaction does not apply to the phenol and phenol ether substrates. Although formyl chloride, a highly unstable intermediate, was previously hypothesised, it is now believed that formyl cation (i.e., protonated carbon monoxide), [HCO]+, reacts directly with the arene without first forming formyl chloride, as opposed to the former hypothesis. Additional requirements include the presence of residues of copper(I) chloride or nickel(II) chloride co-catalyst when zinc chloride is employed as the Lewis acid rather than aluminium chloride, for example, or when carbon monoxide is not utilised at high pressure, among other things. This “carrier” function is performed by the transition metal co-catalyst, which first reacts with CO to produce a carbonyl complex, which is subsequently converted into the active electrophile.
Conclusion
The Gattermann reaction can be thought of as a technique of formylation of aromatic ring compounds, which can be characterised as follows: Additionally, the reaction is referred to as gattermann salicylaldehyde synthesis and gattermann formylation, among other names. The Friedel-Crafts reaction and the Gattermann reaction are both chemical reactions.
Known variously as the Gattermann formylation and the Gattermann salicylaldehyde synthesis, the Gattermann reaction is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst, such as AlCl3.
The reagent employed in the Gatterman reaction is copper powder/hydrochloric acid (HCI).
In the presence of anhydrous aluminium chloride or cuprous chloride, benzene or its derivative is treated with carbon monoxide and hydrogen chloride to produce benzaldehyde or substituted benzaldehyde, which is then converted to benzaldehyde. The Gattermann Koch reaction is the name given to this reaction.
This is the reagent that was employed in the Gatterman Koch aldehyde synthesis: Pb/BaSO4.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



