Due to the presence of a triple bond in alkynes, halogens, water etc.can be added to them by the process of the addition reaction. A series of actions is required to create additional products. The formation of additional products is attributed to the stability of vinylic cations in the presence of water. Asymmetric alkynes must adhere to Markovnikov’s rule in order to undergo an additional reaction, which they do by following the rule.
Addition of alkynes with halogens (halogenation)
Alkynes and halogens undergo an additional reaction to form halogenated alkenes which further react with halogens to give halogen substituted alkanes. The reddish orange coloured solution of bromine and carbon tetrachloride gets decolorized as a result of the addition reaction. This is used as a test for unsaturation.
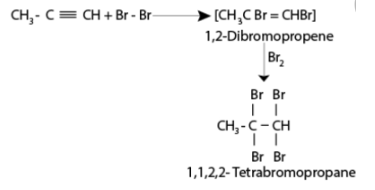
Addition of alkynes in the presence of hydrogens (hydrogenation)
Alkynes react with dihydrogen in the presence of catalysts such as Pt/Pd/Ni to generate alkenes, which are then used to make other compounds. The alkenes that are generated react with dihydrogen to form alkanes, which is the end product. When dihydrogen is added, it has been shown that most reactions result in the conversion of a triple bond into a double bond, and then the conversion of the double bond into a single bond.
Rhodium, nickel, palladium, and platinum are examples of catalysts that are used in the production of these metals. The process of hydrogenation is a step-by-step procedure in which an alkene is first created. After that, it goes through a second hydrogenation process to become an alkane.
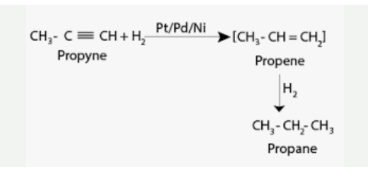
Dihydrogen Alkynes are used in the formulation.
Due to the fact that the entire reaction is extremely smooth, it is nearly difficult to slow down the reaction during the intermediate stage of the reaction. Some alkenes, on the other hand, are separated through the application of poisoned catalysts. Lindlar catalyst is one such example of a poisoned catalyst in action.
Lindlar serves as a catalyst.
Lindlar catalyst is a combination of palladium and quinoline that is deposited on calcium carbonate and then absorbed by the carbonate.
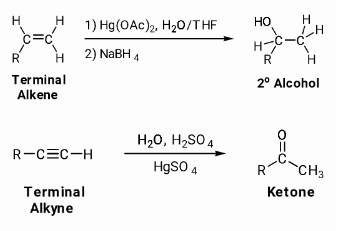
Addition of alkynes in the presence of water (hydration)
Alkynes are insoluble in water because of their chemical structure. Under normal circumstances, they have no reaction with water. At 333K, alkynes can react with water in the presence of dilute sulphuric acid and mercuric sulphate, resulting in the formation of mercuric sulphate. The production of carbonyl compounds is the outcome of this reaction.
Alkynes are combined with hydrogen halides in this process (hydrohalogenation)
When hydrogen halide is treated with alkynes (triple bond compounds), gem halides are formed as a result of the reaction. Gem halides are compounds in which two halogen atoms are linked to the same carbon atoms in a molecule, resulting in a gem halide compound.
Conclusion
The primary reaction of alkynes is addition across the triple bond, which results in the formation of alkanes. Similar to the addition reactions of alkenes, these addition reactions occur in the presence of alkenes. Hydrogenation. Alkynes are hydrogenated catalytically using the same catalysts as alkenes: platinum, palladium, nickel, and rhodium. Platinum, palladium, nickel, and rhodium are the catalysts used in alkene hydrogenation. Hydrogenation occurs in a stepwise fashion, beginning with the formation of an alkene, which then undergoes further hydrogenation to form an alkane.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




