Carbon dioxide is a chemical element that can be found in the atmosphere. At room temperature, it is a gas. It has one carbon atom and two oxygen atoms. When humans and animals exhale, carbon dioxide is released into the atmosphere. It is a greenhouse gas that is present in trace amounts in the Earth’s atmosphere. It is dry ice when it is hard.
Carbon dioxide is emitted into the atmosphere as a result of human activity. When hydrocarbon fuels (such as wood, coal, natural gas, gasoline, and oil) are burned, carbon dioxide is released. During combustion or burning, carbon from fossil fuels combines with oxygen in the air to produce carbon dioxide and water vapor.
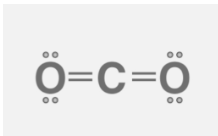
Structure of carbon dioxide
Chemical Properties of Carbon Dioxide’s
Carbonic acid, H2CO3, is formed when carbon dioxide dissolves slightly in water and forms a weak acid termed carbonic acid, H2CO3:
CO2 + H2 → H2CO3
After carbonic acid interacts mildly and reversibly in water, the hydronium cation, H3O+, and the bicarbonate ion, HCO3–, are generated as follows:
H2CO3 + H2O → HCO3– + H3O+
Plants take carbon dioxide (CO2) and water (H2O) from the air and soil during photosynthesis. Water is oxidized in the plant cell, which means it loses electrons, while carbon dioxide is reduced, which means it gains electrons. Water is converted to oxygen, while carbon dioxide is converted to glucose. The plant then releases oxygen into the environment while storing energy in the glucose molecules.
The process through which green plants and other organisms convert light energy into chemical energy is known as photosynthesis. Green plants use light energy to transform water, carbon dioxide, and minerals into oxygen and energy-rich organic molecules during photosynthesis.
Carbon dioxide’s Primary Function
Carbon is the major component of proteins, lipids, nucleic acids, and carbohydrates. Carbon’s molecular structure enables it to bind with a wide range of elements in various ways. The carbon cycle displays how carbon moves through the living and nonliving components of the environment.
Carbon dioxide (CO2) is the most significant greenhouse gas emitted by human activity. Carbon is the major component of proteins, lipids, nucleic acids, and carbohydrates. Carbon’s molecular structure enables it to make bonds with a wide range of elements in various ways. The carbon cycle represents the movement of carbon in the world, including both living and non-living carbon.
Carbon dioxide is required for both photosynthesis and respiration, two critical plant and animal functions. Green plants convert carbon dioxide and water into food molecules such as glucose and oxygen. This process is known as photosynthesis.
The photosynthetic reaction is as follows:
6CO2 + 6H2O → C6H12O6 + 6O2
Carbon dioxide is the fundamental hormone of the body; it is the only one that is released by every tissue and presumably affects every organ. CO2 has numerous roles in the body, including oxygen transport to cells, blood pH management, and many others.
Environmental Issues due to Carbon Dioxide
1.A rise in CO2 levels causes an oversupply of greenhouse gasses, which trap more heat. This trapped heat melts ice caps and raises ocean levels, resulting in flooding.
2.CO2 emissions pollute our pristine air, generating an impenetrable coating all over the world. In a sense, this sheet traps heat inside the earth, generating global warming. This procedure is also known as the Greenhouse effect.
3.Carbon dioxide is released into the environment as a result of the combustion of fossil fuels, the release of chemicals into the atmosphere, the reduction of forest cover, and the rapid expansion of cultivation, production, and industrial activities, all of which alter the climate system’s equilibrium.
4.The Earth’s temperature is determined by a balance between incoming solar energy and energy reflected back into space. Carbon dioxide absorbs heat that would otherwise be lost to space. Any of this energy is re-emitted into the atmosphere, making it even hotter.
Reaction of Carbon With Halogen
A halogen addition reaction is a simple organic reaction that involves the addition of a halogen molecule to the carbon–carbon double bond of an alkene functional group. The halogen addition reaction has the following generic chemical formula-:
C=C + X2 → X−C−C−X.
Conclusion
Carbon dioxide (CO2) is a chemical molecule that exists as an acidic colorless gas with a density that is approximately 53% greater than that of dry air. A carbon atom is covalently doubly linked to two oxygen atoms to form a carbon dioxide molecule.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out