The concept that an electron possesses an intrinsic angular momentum that can be identified as a spin was proposed by S.A. Goutsmit and G.E. Uhlenbeck in 1925. Furthermore, the intrinsic angular momentum of each particle is parametrized by spin quantum numbers in atomic physics. The spin quantum number is also the fourth number, with the magnetic quantum number, primary quantum number, and azimuthal quantum number being the other three.
The spin quantum number explains how an electron’s quantum state is unique. Furthermore, this is confirmed with the letter ‘s’. In quantum mechanics, spins also play a key role in computing the properties of fundamental particles such as electrons.
Spin quantum number
It is the fourth quantum number, indicated by s or ms, and it is the most fundamental of them all. The spin quantum number of an electron in an atom reflects the direction of the electron’s inherent angular momentum within the atom. It defines the quantum state of an electron, including its energy, orbital form, and orientation within its orbital sphere of influence.
‘Spin up’ and ‘spin down’ are the only two possible values of the spin quantum number (also known as the spin quantum number): +12 and -12. The value of spin is a quantum state, and it is not something that can be simply grasped like the direction in which an electron rotates!
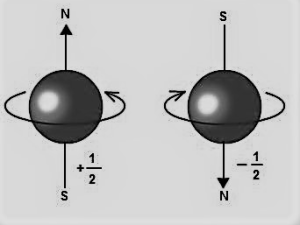
Electron spin quantum number
The quantum property of electron spin is a quantum property of electrons. It’s an example of angular momentum in action. This angular momentum’s magnitude is unchangeable. Spin, like charge and rest mass, is an unchanging characteristic of the electron.
Instructors may compare electron spin to the world revolving on its axis every 24 hours as a teaching approach. Spin-up describes an electron spinning clockwise on its axis, whereas spin-down describes an electron spinning counterclockwise. This is a handy explanation, even if it is not entirely mathematically justified.
The orbital angular momentum associated with electron spin is separate from the spin angular momentum involved with the electron’s passage around the nucleus.
Magnetic field
What source does a magnet’s magnetic field derive from? Moving electric charges generate magnetic fields, which are then reflected back into space. Everything is made up of atoms, and each atom contains a nucleus composed of neutrons and protons, with electrons orbiting around the nucleus. Atoms are the building blocks of matter. Because the electrons in orbit have such minuscule moving charges, a little magnetic field is formed around each atom they pass through. A certain orientation or direction is assigned to each of these magnetic fields, and this orientation is denoted by the term “magnetic moment.” Essentially, all of the atoms in an item behave as if they were a collection of little magnets. In most materials, all of these moments point in random directions and cancel each other out, resulting in a net magnetization of zero, indicating that the item will not act as a magnet when touched. However, when all or most of these moments align in the same direction, the entire item becomes magnetised and generates a magnetic field around itself, which is known as net magnetization.
Conclusion
An electron spin is a type of angular momentum that electrons have. In addition, it is a quantum feature of electrons, and its magnitude is constant. The spin quantum number tells us about an electron’s particular quantum state. In quantum mechanics, spins also play a significant role.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




