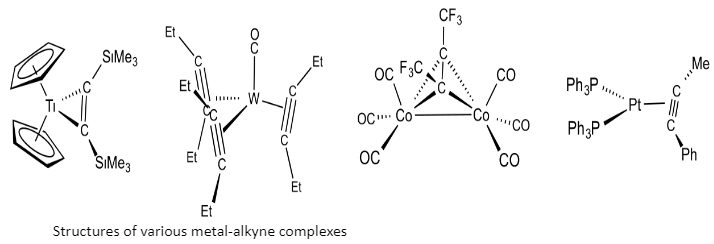
Transition metal alkyne complexes are regularly formed by the way of the displacement of labile ligands by using the alkyne. As an example, the cobalt-alkyne complexes can be formed by the reaction of the alkyne with dicobalt octacarbonyl.
Synthesis
Co2(CO)8+ R2C2 → Co2(C2R2)(CO)6+ 2CO
Many alkyne complexes are produced by using reduction of metal halides:
Cp2TiCl2 + Mg + Me3SiC≡CSiMe3 → Cp2Ti[(CSiMe3)2] + MgCl2
Applications
Metallic-alkyne complexes are also intermediates in the metal-catalyzed trimerization and tetramerizations. Cyclooctatetraene is constituted of acetylene through the intermediacy of metal alkyne complexes. Variations of this reaction are exploited for some syntheses of substituted pyridines.
Alkyne Trimerization
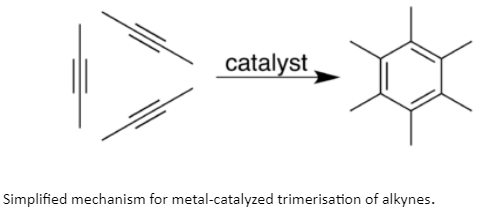
An alkyne trimerisation response is a [2+2+2] cycloaddition reaction wherein 3 alkyne units react to form a benzene ring. This reaction requires a metal catalyst. The manner is of historic interest in addition to being applicable to natural synthesis. Being a cycloaddition response, it has a high atomic economy. Many versions were advanced which include cyclisation of combinations of alkynes and alkenes as well as alkynes and nitriles.
Mechanism
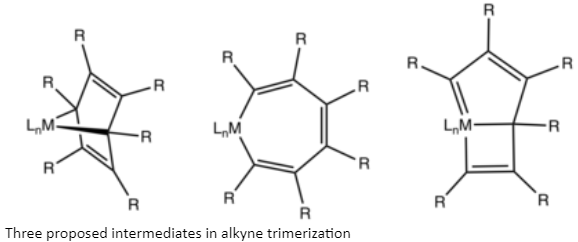
In phrases of mechanism, the reactions begin with the formation of metallic-alkyne complexes. The combination of two alkynes within the coordination sphere provides a metal cyclopentadiene. Beginning from the metal cyclopentadiene intermediate, many pathways can be considered including metallic cycloheptatriene s, metallanorbornadienes, and a extra complicated structure presenting a carbenoid ligand
Catalysts used to include cyclopentadienylcobalt dicarbonyl and Wilkinson’s catalyst.
Stereochemistry and Regiochemistry
Trimerisation of unsymmetrical alkynes gives isomeric benzenes. As an instance, phenylacetylene affords each 1,3,4- and 1,2,4-C6R3H3 .The substitution pattern approximately the product arene is determined in two steps: formation of the metallo cyclopentadiene intermediate and incorporation of the third equivalent of alkyne. Steric bulk at the alkyne coupling partners and catalyst were invoked because of the controlling elements of regioselectivity.
Chiral catalysts are employed in combination with arynes to give non-racemic atropisomeric products.
Synthetic Applications
Trimerisation of three 2-butyne (dimethylacetylene) molecules produces hexamethylbenzene. The reaction is catalyzed by triphenyl chromium tri-tetrahydrofuranyl or by triisobutyl aluminium and titanium tetrachloride.
Metal Complexes of Aldehydes
The metal complexes of aldehydes talks about the coordination of complexes with aldehyde (RCHO) as well as with the ketone (R2CO) ligands. As aldehydes and ketones are common, the area is of great importance. Certain reactions which are useful in organic chemistry involve such complexes.
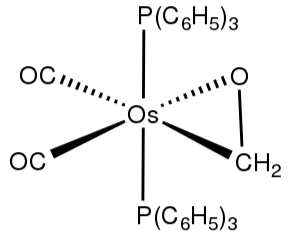
Structure of η2-formaldehyde
In monometallic complexes, aldehydes and ketones bind to metals in modes, η1-O-bonded and η2-C,O-bonded. These bonding modes are mentioned sigma- and pi-bonding. Those forms are sometimes interconverted.
The sigma bonding mode is extra commonplace for higher valence, Lewis-acidic metal centers (e.g., Zn2+)and the pi-bonded mode is located for low valence, electron-rich metal centers (e.g., Fe(zero)).
For the purpose of electron-counting, O-bonded ligands are counted as 2-electron ligands: they are Lewis bases. η2-C,O ligands are also called analogous alkene ligands, which is the Dewar-Chatt-Duncanson model.
η2,O aldehydes can feature as bridging ligands, utilizing a lone pair of electrons on oxygen. One such complex is [(C5H5)2Zr(CH2O)]3, which has a Zr3O3 ring.
Related to η1-O-bonded complexes of aldehydes and ketones are metallic acetylacetonates and associated species, which can be regarded as a mixture of ketone and enolate ligands.
Reactions
A few η2-aldehyde complexes include alkenes to give five-membered metallacycles.
η1-Complexes of alpha-beta unsaturated carbonyls showcase enhanced reactivity towards dienes. This process is on the basis of Lewis acid which is catalyzed by Diels-Alder reactions.
Metal Complexes
Metal complexes basically include central metal ions which are used in multiple reactions and the most common one is photosynthesis, transportation of oxygen into the blood, a few metabolic processes, etc. Biomolecules can even form metal complexes that have their own importance in biomedical. The metal complex and products which are formed using oligo-elements are used in the therapies because they have the properties of pharmacodynamics. The major aspect of the formation of metal complexes by pharmaceutical substances and ligands can be shown in various researches. The usage of ligands and complexes in medicine and biology has multiple uses like antidotes if poisoned. The introduction of metal ions in a living organism is deficient. Bacteria, viruses, and enzymes are important for the body.
Conclusion
- In organometallic chemistry, a transition metal alkyne complex is a coordination compound including one or more alkyne ligands. Such compounds are intermediates in most of the catalytic reactions that convert alkynes to other organic products, e.g. hydrogenation and trimerization.
- One of the characteristic properties of transition-metal compounds is the presence of unpaired electrons in the partially filled d-shell which results in magnetic properties. The metal ions are therefore paramagnetic, which means that they are attracted by an applied magnetic field.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




