The halogen addition reaction has the following chemical formula:
C=C + X2 → X−C−C−X
(X stands for the halogens bromine or chlorine, and the solvent in this example may be CH2Cl2 or CCl4). A vicinal dihalide is the product.
The reaction is a combination of both halogenation and electrophilic addition.
Reaction Mechanism
The following is a description of the reaction mechanism for alkene bromination.
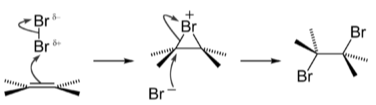
A bromine molecule approaches the electron-rich alkene carbon–carbon double bond in the first phase of the reaction. As the electrons of the double bond resist the electrons of the bromine atom closer to the bond, it gains a partial positive charge.
At this point, the atom is electrophilic and is attacked by the alkene’s pi electrons [carbon–carbon double bond]. It establishes a single sigma bond to both carbon atoms involved for a brief moment. Bromine bonding is unique in this intermediate because, due to its larger size than carbon, the bromide ion can connect with both carbons that earlier shared the -bond, forming a three-membered ring. The bromide ion gains a positive formal charge as a result of this reaction. The halogen ion is now known as a “bromonium ion” or a “chloronium ion,” respectively.
When the first bromine atom attacks the carbon–carbon link, one of its electrons is left behind with the second bromine it was bound to in Br2. That second atom is now a negative bromide anion, attracted to the carbon atoms’ tiny positive charge. The initial bromine atom blocks nucleophilic assault on one side of the carbon chain, so it can only attack from the other side. The link between the first bromine atom and the other carbon atoms breaks when it attacks and makes a bond with one of the carbons, leaving each carbon atom with a halogen substituent.
What are Halogens?
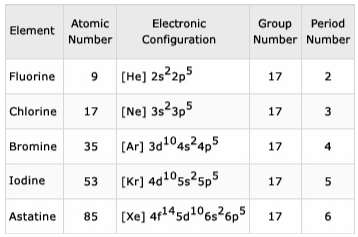
Halogens are extremely reactive and electronegative elements. They have a strong proclivity for forming salts when they come into contact with metals. Group 17 elements are another name for them. In their outer shell, they have 7 electrons in a configuration of (ns2 np5). The first halogen in group 17, fluorine, is extremely reactive. Despite being radioactive, astatine is classified as a halogen due to its similarities to iodine.
This structure clearly demonstrates that their valence shell has 7 electrons. To complete their octet and achieve noble gas configuration, they need one more electron. This explains their high reactivity and proclivity for gaining one electron and forming ionic connections, as well as their tendency to share electrons with other elements and form covalent bonds.
Properties of Halogens
- In nature, halogens are extremely reactive.
- Halogens are not found in nature as pure elements because of their extreme reactivity; instead, they are found as compounds with other elements or as ions.
- Because of their reactivity, most halogens are present in marine and ocean bodies as salts with other elements.
- In nature, halogens are the only elements that exist in all three forms. Chlorine and fluorine are gases at normal temperatures and pressures, while bromine is a liquid and iodine and astatine are solids.
- When halogens react with hydrogen, they produce hydrogen halides, which are powerful acids.
- Halogens can produce diatomic interhalogen compounds when they react with one another.
What Are Organic Reactions?
Organic reactions are chemical processes that involve organic compounds. The most prevalent types of organic chemistry reactions are addition reactions, elimination reactions, substitution reactions, pericyclic events, rearrangement processes, photochemical reactions, and redox reactions. Organic reactions are used in organic synthesis to create new organic molecules. Many man-made chemicals, such as medications, polymers, food additives, and textiles, are synthesised using organic methods.
Combustion of organic fuels and saponification of lipids to generate soap are two of the oldest organic reactions. Wöhler’s synthesis in 1828 marks the beginning of modern organic chemistry. The Nobel Prize in Chemistry has been awarded for the creation of specific organic reactions throughout history, including the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979, and olefin metathesis in 2005.
Functional Groups
In organic chemistry, a functional group is a group of atoms or bonds within a material that is responsible for the compound’s distinctive chemical reactions. The same functional group will behave and experience comparable reactions regardless of the chemical in which it is contained. Functional groups are also important in organic compound naming; combining the names of the functional groups with the names of the parent alkanes allows compounds to be distinguished.
Covalent bonds link the atoms of a functional group to one another and to the rest of the complex.
The alpha carbon is the first carbon atom to attach to the functional group, followed by the beta carbon, gamma carbon, and so on.
Conclusion
Because of their proclivity for forming salts after reacting with metals, the term “halogen” implies “salt-producing. “As we advance along the group, the ionisation enthalpy drops because additional energy levels away from the nucleus are added. Fluorine is an exception, with a lower electron gain enthalpy than chlorine due to its tiny size and greater repulsion. Fluorine is a pale yellow liquid, chlorine is a pale green liquid, bromine is a reddish-brown liquid, iodine is violet, and astatine is a black metallic liquid. Fluorine reacts strongly with hydrogen, chlorine reacts with UV radiation, bromine reacts with heat, and iodine reacts with a catalyst. After interacting with oxygen, halogens produce oxides.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




