This law was named after the French Chemist Gay Lussac, who discovered the relationship between the pressure of a gas and its absolute temperature.
What is Gay Lussac’s Law
Gay Lussac’s law of Thermodynamics state that when the volume is held constant, the pressure of the given mass of gas varies directly with the gas’s absolute temperature. The kinetic energy of the molecules of the gas increases, which in turn hits the walls of the container with greater force, resulting in greater pressure. Mathematically, it can be written as P/T=k. It is a special case of ideal law gas.
This law is also commonly known as the Pressure Law or Amontons’s Law.
Gay Lussac’s Law formula
P∝T (Volume constant)
Removing the proportionality-
P=kT —(1)
(here P= Pressure e
k= constant
T= Temperature)
For Ideal Gas Equation-
PV=nRT —(2)
Substituting the value of P from equation (1) to (2)-
kTV=nRT
k=nR/V
k∝1/V —(3)
From the Gay Lussac’s Law formula, we see when volume increases and k(constant) decreases.
The law can be written as follows for comparing the same substance under two different sets of conditions:
P1 T2 = P2 T1
Graphical Representation of Gay Lussac’s Law
The graphical representation of this law is also known as the P-T graph. The graph is drawn by taking the absolute temperature on the x-axis and pressure on the y-axis. This relation is called the pressure-time relation. The slope in this graph represents k(constant) and is inversely proportional to the volume. Therefore, if we increase the volume, the slope will decrease. Here, it is given that V1 < V2 < V3 < V4 and the graph can be represented as follows:
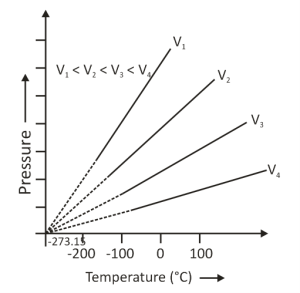
The graph illustrates that gas pressure kept at a constant volume constantly decreases as it cools until the gas eventually condenses and becomes a liquid.
About the Scientist- Gay Lussac
Joseph-Louis Gay Lussac is a French chemist and physicist. He initiated the study into the behaviour of gases and developed new techniques in the analysis and experimentation of the same, which led to notable advances in applied chemistry. He was born on December 6, 1778, in France. Gay Lussac’s first publication came out in 1802, which dealt with the thermal expansion of gases. He used dry gases and mercury for error-free results in the experiment. From the experiment that he performed, he concluded that all gases expand equally in the temperature range of 0-100 ° C. This law is usually attributed to the “Charles Law.” These were the first of several regularities in the behaviour of matter that Gay Lussac established. Gay Lussac’s with his friend Louis Jacques Thenard identified a class of substances (later called carbohydrates) such as sugar and starch that contained a 2: 1 ratio of hydrogen and oxygen. They published the results in three laws, depending on the ratio of hydrogen to oxygen in the substance.
Real-life Examples of Gay Lussac’s Law
- Vehicle Tyre- Vehicles inflated tyres may burst on sweltering summer days. This bursting of tyres is due to Gay Lussac’s Law. There is a lot of pressure inside the inflated tyres, so when the temperature of the air inside the tyre rises, so does the gas pressure in the tube. The tyres fracture after reaching an unbearable point.
- Pressure Cooker- The pressure cooker is a sealed utensil that uses steam pressure to cook food. It is typically made of steel or aluminium. When heat is applied to the cooker, the water inside vaporises, and steam is produced. To maintain the operating pressure inside the cooker, steam is periodically released through a valve. If the valve fails and the heat flow continues, the pressure inside the cooker rises. The pressure rise as stated on the Gay Lussac’s Law. This high pressure may cause the cooker to rupture, resulting in an unfortunate accident.
- Water Heater- Electric water heaters are similar to pressure cookers—the heating filaments inside heat the cold water. The hot water produced is discharged through the outlet nozzle. Modern electric heaters automatically control water temperature. When the system and pressure-relief valve fail, a continuous power supply generates steam. This steam has the potential to damage the heater. The heater may burst if the steam pressure exceeds the tolerable limit.
- Aerosol Cans- Aerosol cans and sprays are devices that spray an aerosol, a suspension of fine solid particles or liquid droplets in the air. The bursting of an aerosol can illustrate gay Lussac’s Law. When an aerosol can is exposed to high temperatures, the propellant vaporises. These vaporised gases exert pressure on the can’s wall. According to Gay Lussac’s Law, the pressure on the wall increases with temperature. Finally, when the pressure becomes unbearable, the can bursts. This is why aerosol cans are recommended to be kept away from heat.
Conclusion
We learned about Gay Lussac’s Law of Thermodynamics. It is an important study to learn about the behaviour of ideal gases. Gay Lussac law has application in real life too. The significance of this gas law is that it shows that increasing the temperature of a gas causes a relative increase in its pressure (assuming that the volume does not change). Similarly, decreasing the temperature causes the pressure to decrease proportionally.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






