An electric cell has two terminals. One is a positive terminal, known as cathode. While the other is a negative terminal, known as the anode. The terminals of a cell are connected with wire to form a closed circuit. Now, when an electric current is drawn from a cell, it flows from the cathode to the anode inside the cell, through the electrolyte. But, in the external circuit, current flows from the anode to the cathode. This mechanism offers some resistance to the flow of current, which we can term as Internal Resistance. So, now we know what internal resistance is. Let us understand its concepts, uses and factors elaborately.
Internal Resistance
Definition – The resistance offered by the electrolyte inside the electric cell to the flow of current is called the Internal Resistance of the cell. The unit of Internal Resistance is Ohm (Ω). A simple device for maintaining a steady current in a circuit is an electric cell. It consists of two electrodes – positive and negative. These are immersed in the electrolyte of the cell. Dipped in the electrolytic solution, these positive and negative electrodes exchange charges with the solution.
During the flow of current, we can notice that the current flows from positive to negative terminal in the external circuit but flows from negative to positive through the electrolyte of the cell. As a result, the electrolyte provides resistance to the current flow. This is Internal Resistance.
The relationship between internal Resistance (r) and e.m.f. (e) of an electric cell in the presence of external resistance (R) across the cell is – e = I (r + R)
We can also write this equation as: e = Ir + IR
The terminal voltage of a cell (V) = current flowing in a conductor (I) Resistance of the conductor (r)
So, the equation for electromotive force changes to: e = V + Ir e = V + v
Similarly, we can derive the formula for Internal Resistance as well.
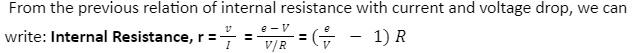
Let us understand the above concepts in a numerical problem:
A cell of e.m.f. 1.5V, internal resistance 1Ω, is connected to the resistors of 18 and 6 in series. Then, calculate the current in the circuit and the voltage drop when the current is flowing.
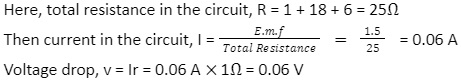
Factors that Affect the Internal Resistance of an Electric Cell
The internal resistance varies from one cell to another, depending on the following factors:
The Surface Area of the Electrodes – Internal Resistance of a cell is inversely proportional to the area of the electrodes dipping in the electrolytic solution. The greater is the surface area of the electrodes of the cell, the lesser is the internal resistance value.
The Distance between the Electrodes – The larger is the distance between two electrodes of the cell, the higher is the value of the internal resistance. This is because the current has to flow through a larger distance of the electrolyte.
The Temperature of the Electrolyte – Internal resistance is inversely related to the temperature of the electrolyte. The more the temperature of the electrolyte, the lesser the internal resistance of the cell. This is because, when the temperature is high, ions easily gain energy. So, the resistance against the charge flow decreases.
The Concentration of the Electrolyte – The higher is the concentration of the electrolyte, the more is its internal resistance.
The Nature of the Electrolyte – Internal Resistance is inversely proportional to the nature of the electrolyte. The more is the conductivity of the electrolyte, the lesser is the internal resistance it offers.
Besides knowing what is Internal Resistance at a depth, we have to learn the concepts for the following:
Electromotive Force – When the electric cell is in an open circuit, the potential difference between the terminals of the cell is what we know as the e.m.f or electromotive force. We can denote the e.m.f of a cell with ‘e’ or ‘𝜺’ (epsilon).The unit for electromotive force is Volt.
Potential Difference – The amount of work done in moving a positive charge from one point to the other is known as potential difference.It is measured in Volt.
Conclusion
With ageing, the internal resistance of the cell increases. Moreover, this causes a voltage drop when current flows through the electrolyte. Both the electrodes and the electrolyte present in the cell offer a resistance, known as the internal resistance of the cell. This resistance opposes the flow of current through the electrolyte of the cell.
So, in this context, we got to know the Internal Resistance definition, how it can be calculated and what factors it depends on.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






