Both mathematics and daily life use sequences and series. The Sequence is a group of numbers arranged in a certain sequence or in accordance with a variety of criteria. A series is created by adding the digits in a sequence.
Items are placed in Sequence in a predetermined order in accordance with certain rules. A specific pattern of numbers is essential in the game of Sequence. The numerical order is very important in the game Sequence.
The components of a series don’t have to be arranged in any specific order. The number pattern in a series is irrelevant. The order in which characters appear in a series is also irrelevant.
For different patterns and Series, there are numerous formulas available. The first term, the nth term, common variables, and other combinations of unknown parameters can all be found using them. There is a unique set of formulas for each kind of sequence and series.
Picture Series and Sequences
Images from reasoning problems based on a series of images, such as a question with five figures in a sequence labelled A, B, C, D, and E. Sequence and series images. These figures are categorized as problems because they demonstrate a progression of change. The five figures that follow the problem are the solutions: 1, 2, 3, 4, and 5. To continue the Sequence started by the problem figures, you must choose one response figure out of a set of five.
Problems based on Picture Series and Sequences
In this kind of query, a selection of images will be provided. The first and last figures are unlikely to be the same in most questions. The requirement is to select the figure from the provided possibilities that will continue the Series.
The first and last photographs in the preceding diagram are identical, so it stands to reason that the following picture would also be identical to the second image in the details. The outcome is that the solution figure matches the second figure exactly.
Solved Examples
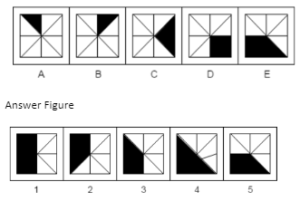
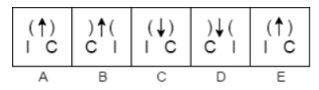
Example 1. Which answer figure will continue the same series as established by the problem figures?
Figure
Answer
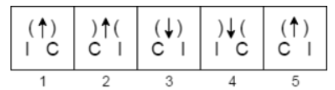
A.1
B.2
C.3
D.4
E.5
Answer
The Correct Answer is B.
In problem figure A, the arcs point in the direction of the arrow, then in the following step, they point away from the arrow, and so on.
Additionally, the arrows are pointing upward in the first two problem figures, downward in the following two problem figures, and so on.
Additionally, the two signals are switching locations alternatively in the lower portion of the issue figures.
Now that the answer figure 2 satisfies all the prerequisites, the series will proceed with it.
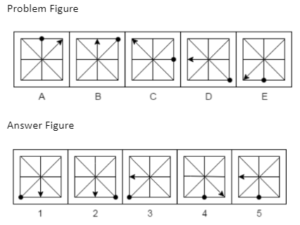
Example 2. Which answer figure will continue the same series as established by the problem figures?
Figure
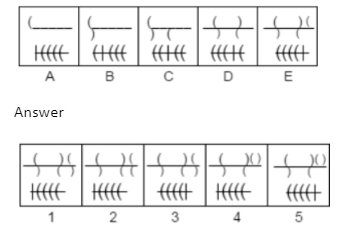
A.1
B.2
C.3
D.4
E.5
Answer
The Correct Answer is A.
In each step, an arc is added to the previous arc in the upper portion of the problem figures to create the shape of the letter S. Additionally, the vertical line in the lower portion of the issue figures advances one step with each step. As a result, the series will resume with response figure 1.
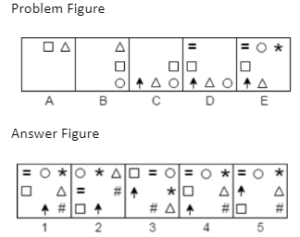
Example 3. Which answer figure will continue the same series as established by the problem figures?
Figure
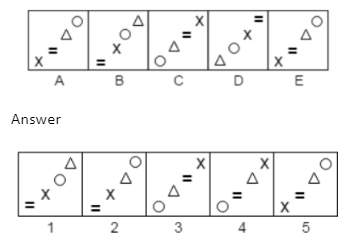
A.1
B.2
C.3
D.4
E.5
Answer
The Correct answer is A.
The pairs of signs (x, =) and (∆, O) first switch places, and then the positions of both pairs are switched. As a result, the series will resume with response figure 1.
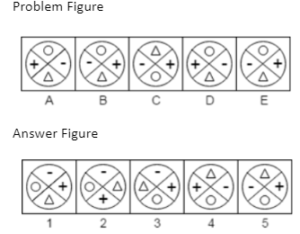
Example 4. Which answer figure will continue the same series as established by the problem figures?
Figure
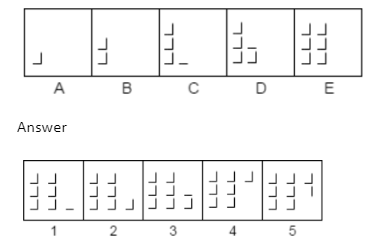
A.1
B.2
C.3
D.4
E.5
Answer
The Correct Answer is B.
L is formed in a predetermined order by adding alternately two and three tiny lines. Therefore, the series will continue with response figure 2.
Conclusion
Through the study material notes on Picture Series and Sequences, we addressed a Series of images and sequences, formulas connected to sequence and series, sequence and series pictures, and other related issues. For your benefit, we also covered Geometric Sequences and Series Formulas and Arithmetic Sequence and Series Formulas.
Finding the following number in the Series is a fun puzzle to solve. A number series is given in these problems. There is a pattern to this numerical sequence. It is necessary to identify this pattern and determine the Series’ subsequent stage. Comparable to pattern image or picture puzzles are number puzzles.
Here, pictures are used instead of numbers. A specific image will be viewed, and a solution with a particular pattern will be discovered. To determine what will happen next in the pattern, the pattern must be visually comprehended. The ability to understand visual data is a non-verbal ability.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out