A blackbody is a perfect radiator which absorbs radiation. The famous equation named after Max Planck was developed to find a relation between the radiation emitted by a blackbody and its temperature and wavelength. He received the prize in 1918 for his work on quantum theory.
He presented the theoretical derivation of the equation that described the blackbody radiation curve on December 14, just two months after he first reported his findings.
Classical physics can’t explain the observed spectrum of radiation. Classical physics assumes that radiation is emitted continuously by matter , with a smooth spectrum of energy levels. In 1900, Max Planck said that the energy is emitted by quanta and not continuously.
When there is no net flow of matter or energy, the unique and characteristic wavelength of the radiation is described by the law. Everybody emits radiation and the power of the radiation per unit area and solid angle is described.
The relationship between the total radiated energy of a body and the peak of the emitted spectrum is shown by the Planck law.
The amount of radiation that a body can emit from its surface is called the Planck radiation. The ratio of the actual radiance to the theoretical Planck radiance is known as the emissivity. It is dependent on chemical composition, temperature, wavelength, angle of passage, and on the polarisation. The emissivity of a natural interface is always between 0 and 1.
Black Body Radiation
A black-body absorbs and emits radiation. The emitted radiation is described by the law of Planck’s law, which states that the higher the temperature of a body , the more radiation it emits at every wavelength.
The maximum intensity of the radiation depends on the body’s temperature.
A black body that absorbs all the radiation incident upon it. A small hole in the wall of a large enclosure can be used to model the black body’s surface. The radiation inside the enclosure is described by the law of equilibrium.
Every body emits radiation. The emitted radiation is almost described by the law. Thermal radiation is said to be the result of its dependence on temperature. The higher the temperature, the more radiation there is. At every wavelength, it emits. The maximum intensity of the radiation depends on the temperature.
For example-A body emits thermal radiation that is mostly invisible at room temperature. The amount of radiation that can be felt as heat increases and more visible radiation is emitted , so the body glows red. The body is bright yellow or blue-white and emits a lot of short wavelength radiation.
What is Planck’s Equation?
Max Planck discovered a theory that energy is transferred in the form of chunks called quanta. The constant value of h is equal to 6.63 x 10-34 J.s and the variable describes the Frequency. The energy of photons is calculated when their frequencies are known.
If the wavelength is known, you can use the wave equation to calculate the energy and then apply the equation of Planck’s equation. The relevancy of the energy per quantum is described by Plank’s constant.
Planck’s Law
It states that the radiation from heated bodies is made up of quanta of energy, which is a fundamental physical constant, and not a continuous flow.
Mathematically,
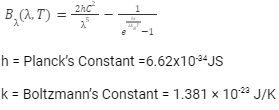
Conclusion
According to the topic ,The energy of photons is calculated when their frequencies are understood. If the wavelength is understood, you can use the differential equation to calculate the energy and then apply Planck’s equation. Different atoms and molecules can emit and absorb energy in different quantities. The smallest amount of energy that can be absorbed is known as quantum.
Quanta of energy is the form of small packets of energy. The energy of each quantum is determined by the amount of radiation. It is proportional to where v is. Frequency.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



