A heat engine is a system that transforms heat into mechanical energy. Mechanical energy is used by various machines to work. The heat engine produces work from heat and rejects some heat.
Heat is rejected as the by-product of the process, which is the fundamental law of thermodynamics. It is why heat engines cannot work with 100% efficiency.
There are three main parts of a heat engine – hot reservoir, system, and cold reservoir. Heat engines are used in various machines such as jet engines, steam turbines, and gasoline and diesel engines. Heat engines have made human travel easy and convenient. With heat engines, we can travel across the world in hours. Let’s discuss how a heat engine powers a machine, the capacity of a heat engine, the parts of a heat engine, and how a heat engine produces energy.
Heat engine
In terms of engineering and thermodynamics, a heat engine is an engine that converts heat to mechanical energy, like that used in a motor vehicle or a steam engine. Mechanical energy can be used in machines. The working principle of all heat engines is the same.
The process in which the end result and the initial input are the same is a cyclic process. In this process, there is a sequence that includes work and heat transfer in and out of the system with the help of different state variables, temperature, and pressure that go back to their original state after the process. The entropy change is zero in the process. This is why it is called a cyclic process. This process gives energy to the machines. The process is fully explained under the title the Carnot heat engine.
There are two types of heat engines:
- External combustion engine
- Internal combustion engine
Parts of a heat engine:
- Hot reservoir: Hot reservoir is the source of heat. The heat needed by the system is provided by the hot reservoir.
- System: In the system, heat mixes with other gases or air that is a working substance, and the system heats up. The heat made in a system can be used in machines to work.
- Cold reservoir: The heat that is left after the use of heat is sent to the sink, which is the cold reservoir. In other words, the cold reservoir absorbs the remaining heat from the system.
The capacity of a heat engine
If
Given heat = Q1
Rejected heat = Q2
Work done in a heat engine (W) can be calculated by:
W = Q1 – Q2
The efficiency of the heat engine can be calculated by:
The efficiency of heat engine = WQ1
Carnot heat engine
The Carnot heat engine is a heat engine developed by Sadi Carnot that works on the principle of the Carnot cycle. The primary principle of the model is that the heat engine works as an energy transferring system from a hot to a cold reservoir, while converting some of the energy into mechanical work. The cycle can also be overturned to work as a heat pump instead of a heat engine. With the help of an external force, the thermal energy from the cool reservoir to the hot reservoir can be converted, working as a refrigerator. This is the most efficient theoretically possible engine, given that the efficiency will depend on the temperature of each of the reservoirs.
There are three parts in the Carnot engine:
- A non-conducting stand so that heat can not be transferred
- A hot reservoir that has infinite heat capacity, so that whatever energy we take, the temperature of the hot reservoir remains constant. It is also called a heat supplier.
- The cold reservoir also has infinite heat capacity, so that whatever energy is given to the cold reservoir, its temperature remains constant. It is also called a heat sink.
- A cylinder to keep the working substance in it. The working substance can be any gas or liquid. A movable piston is fitted with the cylinder. The cylinder and piston wall are made of a non-conducting material.
Process of Carnot engine
A Carnot engine goes through four stages of the Carnot cycle. The cylinder is put in a hot reservoir. Since the temperature of the reservoir is higher than the temperature of the gas, heat flows from the hot reservoir to the gas. Due to this, the gas expands. Because the gas is expanding, the temperature of the gas cannot rise. This process is called isothermal expansion. The volume of the gas increases and pressure decreases.
Then, the cylinder is put on the insulating stand. When doing so, the gas is confined to the non-conducting wall. Now, the piston is slowly risen upward. Due to this, the gas expands. But this time, the gas does not get heat from somewhere else. This type of expansion is called adiabatic expansion. As the expansion is happening without heat, the temperature of gas decreases. The volume of the gas increases and the pressure of the gas decreases.
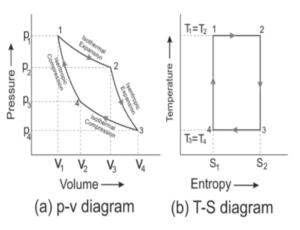
Next is isothermal compression. In this, heat transfers from the system to the cold reservoir due to the surrounding and pressure shifts from P4 to P3. The volume of the gas decreases.
In the final step, i.e., adiabatic compression, there is no addition or subtraction of heat from the system, and the piston moves in the downward direction due to the environment. With the piston continuously moving downward, there is a compression of the gas. The temperature increases and pressure increases from P3to P1. This process increases the gas’ internal energy. The gas compression and temperature increase. After this process, the repetition of the cycle begins from the 1st stage.
This complete cycle is called the Carnot cycle and the engine is called the Carnot engine.
The efficiency of Carnot engine = 1 – T1T2
The efficiency of the heat engine depends on both reservoirs. To make the heat engine 100% efficient, we need to make the hot reservoir’s temperature infinite Kelvin or the cold reservoir’s temperature zero Kelvin. But it is not possible to do so, which is why the Carnot engine is a theoretical engine.
Conclusion
Mechanical energy is used by various machines to work. The heat engine produces work from heat and rejects some heat. Heat is rejected as the by-product of the process, which is the fundamental law of thermodynamics. It is why heat engines cannot work with 100% efficiency. There are three main parts of a heat engine – hot reservoir, system, and cold reservoir. Heat engines are used in various machines such as jet engines, steam turbines, and gasoline and diesel engines. Heat engines have made human travel easy and convenient.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



