You require energy to perform a task. This requirement comes in a variety of forms. Chemical energy is the energy released by chemical substances when they undergo a reaction and evolve into something else. The breaking and remaking of a bond requires energy, which can be absorbed or released by a chemical system. It’s a type of potential energy that isn’t visible until a reaction occurs. Chemical processes are responsible for converting one form of energy to another. The heat absorbed or released in the form of energy during transformation depends on the arrangement of atoms.
Types of Chemical Energy
Substance stored energy and the energy released during the breakdown of a substance are two types of energy. They are grouped under:
- Chemical Potential Energy
- Chemical Kinetic Energy
Chemical Potential Energy
This form of energy is stored in a substance. It is visible when exposed to any chemical reaction and leads to the flow of energy from one state to another. For example, batteries, fossil fuels, etc.
The extracted energy from the substance follows the law of conservation of energy which states that energy can neither be created nor destroyed but can only change form. The arrangement of atoms in a substance leads to the evolution of the substance and releases chemical energy. For example, the food we consume is produced by photosynthesis and is later converted to ATPs, which provide life to creatures.
Chemical Kinetic Energy
The form of energy is gained during motion. The energy released during the reaction is kinetic chemical energy, i.e. exothermic or endothermic reaction.
- Exothermic reaction: This chemical reaction gives heat as a by-product. The reaction tends to increase the overall temperature.
- Endothermic reaction: This type of reaction absorbs heat from the surroundings to complete the evolution of the substance. For example, heat energy is absorbed and processed into carbohydrates in photosynthesis.
Examples of Chemical Energy
Chemical energy is stored in all compounds and can be used to create a new substance when the bonds are broken. Chemical energy can be found in a variety of materials, including:
- Coal: The burning of coal gives heat and light energy.
- Petroleum: Combustion gives heat and light energy.
- Batteries: Chemical energy is converted into electrical energy.
- Biomass: Light and heat energy are gained through the combustion reaction.
- Photosynthesis: Conversion of heat energy into carbohydrates.
- Cellular respiration: Conversion of glucose energy mixed with oxygen to form ATPs.
Role of Energy During the Breaking of Bonds
Chemical compounds that have broken bonds release energy when they create new chemical bonds. As a new material evolves, the energy is released in the form of heat. For example, methane releases carbon dioxide and water when burned in a power plant with oxygen. Burning methane causes the hydrogen atoms of the hydrocarbon to combine with oxygen and form water molecules. Meanwhile, the carbon atoms of the methane react with oxygen to form molecules of carbon dioxide. Combustion breaks the hydrogen-oxygen bonds of methane and forms water molecules and carbon dioxide in the presence of oxygen. Thus, energy is released to form new bonds after breaking old methane bonds.
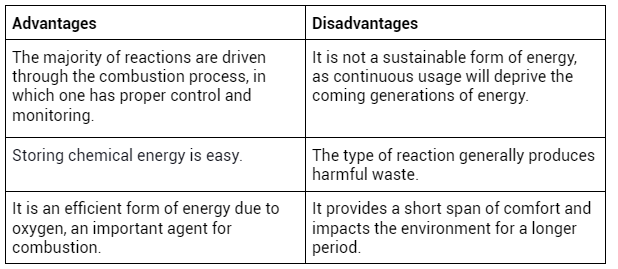
Advantages and Disadvantages of Chemical Energy
Conclusion
Chemical energy is used to perform a specific task achieved by breaking down the chemical bonds and converting one material to another through a particular arrangement of atoms. Exothermic or endothermic reactions are by-products of the conversion. Extracted energy from a substance follows the law of conservation of energy which states that energy can neither be created nor destroyed but can only change form.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




