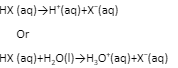Acids are the compounds that turn blue litmus paper red and release dihydrogen when they react with certain metals. Different scientists gave various theories on bases of which substances were classified as acids or bases.
Following are the various theories:
- Arrhenius Concept: Acids produce hydrogen ions in their aqueous solution, while bases make hydroxyl ions, according to Arrhenius.
- Bronsted-Lowry concept: Acid is a proton donor, whereas a base is a proton acceptor, according to Bronsted-Lowry.
- Conjugate pair of acid and base: The sole difference between an acid and a basic conjugate pair is one proton.
- Conjugate base and Conjugate acid: When a Bronsted-Lowry acid interacts with a base, the conjugate base and conjugate acid are formed.
- Lewis’s acids: Define an acid as an acceptor of electron pairs and a base as a donor of electron pairs.
Arrhenius Concept of Acids and Bases
Acids and Bases in the Arrhenius Theory Acids are compounds that break down in the water to produce hydrogen ions H+ (aq). Acids, according to Arrhenius, raise the concentration of H+ ions in water.
For HX (aq), the following equations can be used to represent acid ionisation:
The Arrhenius theory states that acids are substances that dissociate in water to produce electrically charged atoms or molecules called ions, one of which is a hydrogen ion (H+), and that bases ionize in water to produce hydroxide ions (OH). It was proposed by the Swedish scientist Svante Arrhenius in 1887.
The Arrhenius theory states that acids are substances that dissociate in water to produce electrically charged atoms or molecules called ions, one of which is a hydrogen ion (H+), and that bases ionize in water to produce hydroxide ions (OH–). It was proposed by the Swedish scientist Svante Arrhenius in 1887.
The Bronsted-Lowry Acids and Bases
An acid is a substance capable of giving a hydrogen ion H+, while a base is a substance capable of absorbing a hydrogen ion H+, according to Bronsted-Lowry. Acids are proton givers, whereas bases are proton acceptors.
The Arrhenius definition was extended by the lowry theory of acid and bases, which eliminated the need for a substance to have hydrogen (H+) or hydroxide (OH–) ions to be categorised as an acid or basic. Consider the following chemical equation as an example.
![]()
Ammonia (NH3) receives a proton (H+) from hydrochloric acid (HCl), generating a positively charged ammonium ion (NH4+) and a negatively charged chloride ion (Cl–). As a result, HCl is a Brnsted-Lowry acid (it contributes a proton), whereas ammonia is a Brnsted-Lowry base (it accepts a proton) (accepts a proton). Cl– is also referred to as the conjugate base of the acid HCl, while NH4+ is referred to as the conjugate acid of the base NH3.
An acid, like the Arrhenius theory, is a chemical that can release a proton, while a base is a substance that can take a proton. In water, a basic salt, such as Na+F–, creates OH– ions by absorbing protons from the water (to make HF)
Conjugate pair of acid and base
A conjugate acid-base pair is defined as an acid and a base that differ only in the presence or absence of a proton. As a result, NH3 is referred to as the conjugate base of NH4+, and NH4+ is referred to as the conjugate acid of NH3. F– is the conjugate base of HF, and HF is the conjugate acid of F–. Whenever a Bronsted-Lowry acid interacts with a base, the conjugate base and conjugate acid are produced. A conjugate acid-base pair is an acid-base pair that differs only by one proton. Consider the ionisation of HCl in water as an example. Because it receives the proton, water behaves as a base. HCl is the conjugate acid of base Cl, and Cl is the conjugate base of HCl. H2O is the conjugate base of the acid H3O+, while H3O+ is the conjugate acid of the base H2O.
Conjugate base and Conjugate acid
The conjugate acid has one more hydrogen atom and one more positive charge than the base from which it was created. A conjugate base has one fewer hydrogen atom and one more negative charge than the acid from which it was created. Take the reaction of bicarbonate ions with water to produce carbonic acid and hydronium ions as an example. A conjugate acid has one more hydrogen atom and one more positive charge than the base from which it was created.
A conjugate base has one fewer hydrogen atom and one more – charge than the acid from which it was created.
Lewis’s acids
According to Lewis, an acid is a chemical that receives electrons, and a base is a substance that donates them. Lewis’s acids are electron-deficient species such as AlCl3, BH3, H+, and others, whereas Lewis bases are electron-donating species such as H2O, NH3, and others.
Lewis acids are chemical compounds with vacant orbitals that can accept electron pairs from Lewis bases. Chemical entities with a trigonal planar structure and an empty p-orbital were previously referred to as trigonal planar species. An electron pair donor is a Lewis base, while an electron pair acceptor is a Lewis acid. A process of breaking bonds and generating new ones culminates in an organic transformation (the synthesis of products from reactants). This is essentially an electron pair transfer process. The Lewis acid-base theory describes ionic mechanisms because they include electron pair exchanges. Lewis bases have high electron density centres, while Lewis acids have low electron density centres, according to the Lewis definition. The electron pair given by the base is used to create a new sigma bond to the electron-deficient site in the acid in a reaction between a Lewis acid and a Lewis base.
Conclusion
The Acids-Theories Based on the Concept of Acids Chemistry proves or differentiates the acids on differing aspects varying among the theories provided by the scientists. The acid’s properties are described. The Arrhenius theory states that acids are substances that dissociate in water to produce electrically charged atoms or molecules called ions, one of which is a hydrogen ion (H+), and that bases ionize in water to produce hydroxide ions (OH).
H2O is the conjugate base of the acid H3O+, while H3O+ is the conjugate acid of the base H2O.
Lewis acids are chemical compounds with vacant orbitals that can accept electron pairs from Lewis bases. Chemical entities with a trigonal planar structure and an empty p-orbital were previously referred to as trigonal planar species. An electron pair donor is a Lewis base, while an electron pair acceptor is a Lewis acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out