In chemistry, equivalent weight is the amount of a material that perfectly interacts with, or is equal to the combining value of, an arbitrarily set amount of another substance in a certain reaction. Substances react in stoichiometric, or chemically equivalent, amounts with one another, and a common standard is established. The idea of comparable weight has given way to the concept of molar mass, which is the mass of one mole of a material.
The equivalent weight of an element is calculated by dividing its gram atomic weight by its valence (combining power). Silver (Ag), 107.868 grams (g); magnesium (Mg), 24.312/2 g; aluminium (Al), 26.9815/3 g; and sulphur (S, in the form of a sulphide), 32.064/2 g are some comparable weights. The equivalent weight of an element is the amount that manages to combine with or replaces 1.008 g of hydrogen or 7.9997 g of oxygen, or the weight of an element liberated in an electrolysis (chemical reaction caused by an electric charge) by the passage of one faraday (96,485.3321233 coulombs) of electricity.
The equivalent weight for compounds that acts as oxidising or reducing agents (acceptors or donors of electrons) is the gram molecular weight divided by the number of electrons lost or gained by each molecule—for example, potassium permanganate (KMnO4) in acid solution, 158.038/5 g; potassium dichromate (K2Cr2O7), 294.192/6 g; and sodium thiosulfate (Na2S2O35H2O), The equivalent weight of all oxidising and reducing agents (elements or compounds) is the weight of the substance associated with the loss or gain of one mole (6.023 1023) of electrons.
The equivalent weight of an acid or base for neutralisation reactions or of any other compound that acts by double decomposition is the amount of the compound that will furnish, react with, or be equivalent to 1.008 g of hydrogen ion or 17.0074 g of hydroxide ion—for example, hydrochloric acid (HCl), 36.461 g; sulfuric acid (H2SO4), 98.078/2 g; sodium hydroxide (NaOH), 40 g
What Exactly Are Moles?
One mole of a material is defined as 6.02 1023 unique particles (atoms or molecules). (This is the precise number of atoms found in 12 grams of carbon.) The mass of one mole of a specific element, or its molecular weight (MW), is indicated in the appropriate box for that element, generally at the centre bottom, as you proceed from left to right and down on the periodic table.
An example clarifies the meaning of this word. With only one molecule of water, H2O, you can observe how two H atoms combine with one O atom to generate this chemical. However, because the MW of H is around 1.0 and that of O is 16.0, the molecule includes 2(1) = 2 mass parts of H for every (1)(16) = 16 mass parts of O. Thus, only 2/18 = 11/1 of the mass of water is made up of H, whereas 16/198 = 88.9 percent is made up of O.
What Exactly Is Equivalent Weight?
The equivalent weight is the weight (or mass) of a material that contains a single reactive proton (or hydrogen ion, H+) or a single reactive hydroxide ion (OH). The first instance is true for acids, which are proton givers, while the second is true for bases, which are proton acceptors.
The idea of equivalent weight is required because certain compounds may give or absorb more than one proton, implying that the material is doubly reactive for every mole present.
The formula for the general number of equivalents is
E = MW/number of charges
Where MW is the compound’s molecular weight, and charge number is the number of proton- or hydroxide-equivalents it possesses. Examples with various acids and bases demonstrate how this works in practice.
Acid and base equivalents
Consider the following sulfuric acid example:
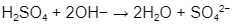
The MW of the acid may be calculated by using a periodic chart to get the MW of each element and then adding 2(1) + (32) + 4(16) = 98.0.
Because the sulphate ion has a charge of 2, this acid can give two protons. 98.0/2 = 49.0 is the equal weight.
The argument is the same for a base. In solution, ammonium hydroxide may receive a proton to form an ammonium ion:
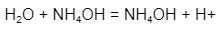
Ammonium hydroxide has MW of (14) + (4)(1) + (16) + 1 = 35.0. E for this molecule is 35.0/1 = 35.0 since just one proton is consumed.
Equivalent mass expressions (EM)
An element’s EM = Atomic mass/Valency
(ii) Acid EM = Molecular mass/Basicity
(The amount of replaceable hydrogen atoms in one acid molecule is referred to as the acid’s basicity.)
(iii) Base EM = Molecular mass/Acidity
(A base’s acidity is defined as the number of replaceable–groups in one molecule of the base.)
(iv) A salt’s EM = Formula mass / Total positive or negative charge
(v) Electromagnetic spectrum of an oxidising agent
= Formula mass divided by the number of electrons obtained per molecule or the total change in O.N.
The equivalent mass of a typical oxidising agent varies with the reaction media.
Conclusion
The equivalent weight of a chemical might change depending on the sort of reaction it goes through. Thus, potassium permanganate reacting by double decomposition has an equivalent weight equal to its gram molecular weight, 158.038/1 g; as an oxidising agent, it can be reduced to the manganate ion (MnO42), manganese dioxide (MnO2), or manganous ion (Mn2+), with equivalent weights of 158.038/1 g, 158.038/3 g, and 158.038/5 g. The number of equivalent weights of any component dissolved in one litre of solution is referred to as the solution’s normalcy.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






