A chemical bond is an atom-to-atom attraction. This attraction can be explained by differences in the behaviour of atoms’ outermost or valence electrons. These actions blend effortlessly into one another in a variety of situations, leaving no visible distinction between them. Differentiating between distinct kinds of relationships, which result in diverse characteristics of condensed matter, is still relevant and common.
Importance of Chemical bonding:
Chemical bonding aids in the combining of atoms or molecules. It also assists components with the same or other substances in forming bonds with one another. Chemical bonding allows solid, liquid, and gaseous matter to exist in nature.
Any of the connections that contribute for the combination of atoms into macromolecules, ions, crystals, as well as other stable organisms that make up the recognisable compounds of everyday life are referred to as chemical bonding.
When atoms come close together, their nuclei and electrons contact and tend to disperse themselves in the system in such a way that the amount of energy is lower than in any other configuration. When the overall energy of a collection of atoms is less than the sum of the strengths of the component atoms, they bind together, and the energy difference is known as the bonding energy.
Types of Chemical Bonds
- Ionic Bond
- Covalent Bond
- Metallic Bond
1.Ionic Bond:
The electrostatic interaction between positive and negative ions in a chemical molecule forms an ionic bond, also known as an electrovalent bond. A bond is created when the valence (outermost) electrons of one atom are irreversibly transferred to another atom.
Ionic bonds are formed by a cation and also an anion. The bond is formed when an atom, often a metal, releases one or more electrons and transforms into a positive ion, or cation. A non-metal atom can accept the electron(s) needed to create a negative ion, or anion.
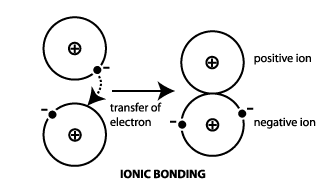
Examples of Ionic Bond
- LiF – Lithium Fluoride
- LiCl – Lithium Chloride
- LiBr – Lithium Bromide
- LiI – Lithium Iodide
- NaF – Sodium Fluoride
- NaCl – Sodium Chloride
- NaBr – Sodium Bromide
- NaI – Sodium Iodide
- KF – Potassium Fluoride
- KCl – Potassium Chloride
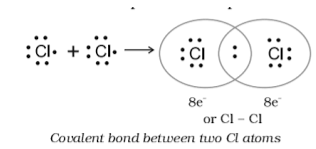
2.Covalent bond
A chemical link involving the exchanging of electron pairs among atoms is known as a covalent bond. Shared pairs or bonding pairs are the permanent balance of attractive and repulsive forces between atoms when they share electrons, while covalent bonding is the stable balance of attraction and repulsion between atoms when they share electrons.
When two or more atoms exchange one or maybe more pairs of electrons, they create a covalent connection. At the same time, these particles are collected to both atomic nuclei. When the difference in electronegativity of two or more elements is too tiny for an electrostatic interaction to produce ions, a covalent bond is established.
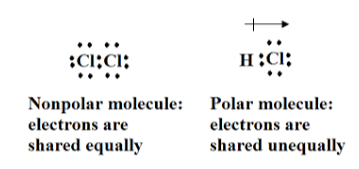
- Polar covalent bond:
The electrons in a polar covalent bond are unequally shared by the atoms, and they spend more time near to one than the other. Slightly positive (+) and slightly negative (–) charges form in different sections of the molecule due to the uneven distribution of electrons between both the atoms of different elements.
ii . Non-Polar Covalent Bond:
A nonpolar covalent link is a sort of chemical connection that occurs when two atoms share electrons evenly. As a result, the number of electrons distributed by neighbouring atoms in an atom would be the same. Because the electronegativity difference is usually minimal, the covalent bond sometimes is referred to as nonpolar.
3.Metallic Bond:
Metallic bonding is a type of chemical bonding that occurs when positive charged ions and conductance electrons attract each other electrostatically. It may be defined as the sharing of delocalized electrons in a structure by positively charged ions (cations). Metallic bonding determines several physical properties of metals, including hardness, ductility, thermal and electrical resistance and permeability, opacity, and brightness.
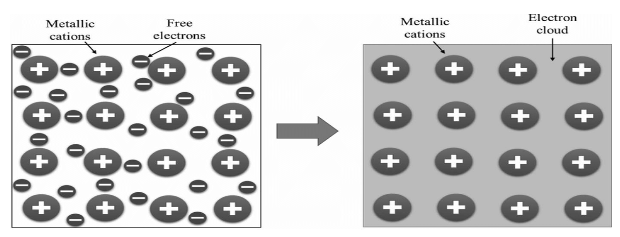
Chemical Bonding and Molecular Structure
A chemical bond is an atom-to-atom attraction. This attraction can be explained by differences in the behaviour of atoms’ outermost or valence electrons. These actions blend effortlessly into one another in a variety of situations, leaving no visible distinction between them. Differentiating between distinct types of bonds, which result in diverse condensed matter characteristics, is still relevant and common.
Molecular Structure:
The placement of the atoms, not really the electrons, is described by molecular structure. We call the geometry that contains all pairs of electrons the electron-pair geometry to distinguish between these two circumstances. The molecular structure is the structure that solely comprises the location of the atoms in a molecule.
Conclusion:
Chemical bonding is the attracting force that keeps diverse components (atoms, ions, and so on) together and stabilises them by causing them to lose energy. As a result, the strength of chemical bonds between constituents determines the stability of chemical compounds; the stronger the connection between constituents, the more permanent the final complex.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






