There are four controlling assumptions for a vapour to be “ideal”: The volume of the gas particles is insignificant. The gas particles are all the same size and there are no forces (attraction or antagonism) between them.
What is an Ideal Gas?
A theoretical gas composed of a huge number of spontaneously moving individual atoms with no interparticle interactions is known as an ideal gas. The ideal gas theory is useful because it satisfies the ideal gas law, which is a simplified equation of conditions that can be analysed using statistical mechanics.
A hypothetical gas composed of components that follow a set of criteria is referred to as an “ideal gas.”
- Gas molecules in ideal conditions are neither attracted nor repulsive to one another. When perfect gas particles impacted each other and with the container’s walls, the only link between them would’ve been an elastic collision.
- Ideal gas molecules have no volume of their own. Because the molecules extend throughout a broad span of space, the gas takes up the volume, however, the Ideal gas molecules are represented as point particles with no quantity in and of themselves.
Equation of Ideal Gas Law:
The macroscopic and microscopic factors such as pressure, volume, and temperature determine the condition of an ideal gas.
Where;
- P is the ideal gas’s pressure.
- V is the ideal gas’s volume.
- n is the mole equivalent of the quantity of ideal gas.
- The gas constant is R.
- T stands for temperature.
Let’s write the definition and units of the each variables;
Pressure (P): It is a normal force per unit area.
Units -N/n²
Volume (V): The amount of space in a three-dimensional closed surface.
Units: m³
Number of moles(n): Moles can be calculated by the following formula;
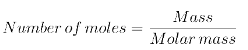
Units: Mole
Ideal gas constant(R): It is the relation between the Pressure in kPa Volume in Liters amount in moles and temperature in Kelvin.
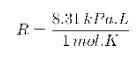
Temperature(T): It is a measure of heat.
Units: Kelvin or Celsius.
This equation is derived from
- Boyle’s law: a law indicating that the pressure of a constant amount of an ideal gas at a constant temperature is inversely proportional to its volume.
- Charles’s law: a law stating that the quantity of an ideal gas at constant pressure is directly proportional to the temperature.
- Avogadro’s law: a statement that equal volumes of various gases contain an equal number of molecules under the same temperature and pressure conditions. After combining three laws we get
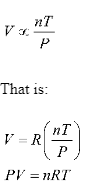
Types of Ideal gases:
There are three basic types of an ideal gas:
- the conventional or Maxwell-Boltzmann ideal gas
- the ideal quantum Bose gas, which is made up of bosons; and
- the ideal quantum Fermi gas, which is made up of fermions.
There are two types of classical ideal gases: the classical thermodynamic ideal gas as well as the ideal quantum Boltzmann gas. The conventional thermodynamic ideal gas is predicated only on classical thermodynamics, and important thermodynamic quantities such as entropy are only given to within an indeterminate additive constant.
The ideal quantum Boltzmann gas solves this problem by specifying these additive constants using the quantum Bose and quantum Fermi gas limits in the high-temperature limit.
Limitations of Ideal Gas
The concept of an ideal gas is founded on false assumptions.
1 Although an ideal gas does not exist in reality, the ideal gas equation is tremendously useful in understanding how gases behave during reactions.
2 Gases that have a low density, low pressure, and high temperature behave similarly to an Ideal Gas.
What is real gas?
A genuine gas is one that does not obey gas laws under all conventional pressure and temperature circumstances. The gas deviates from its ideal behaviour as it becomes bigger and more voluminous. True gas velocity, weight, and volume are all present.
The real gas law equation is given as
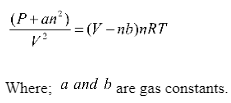
Difference between ideal and real gas
Real gas | Ideal gas | |
Definition | It is present in nature. | Does not exist in nature. |
Intermolecular forces | Present in between the molecules. | Not present in between the molecules. |
Volume | Particles have definite volume. | Particles do not have a specific volume. |
Mass | Particles have definite mass | Particles does not have specific volume |
Kinetic energy | It changes with molecules collision. | It is constant |
Movement between molecules. | Molecules interact with each other. | Molecules do not interact with each other. |
Conclusion:
An ideal gas has no intermolecular forces of attraction and all atom-to-atom or molecule-to-molecule collisions are completely elastic. There are no forces between the gas particles because they are all the same size. An ideal gas is a theoretical gas made up of a large number of spontaneously moving individual atoms with no interparticle interactions. Because it fulfils the ideal gas law, which is a simplified equation of circumstances that can be analysed using statistical mechanics, the ideal gas theory is beneficial.An “ideal gas” is a hypothetical gas made up of components that fulfill a set of requirements.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






