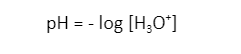Many theories and concepts exist regarding the classification of acids and bases. In earlier times, substances that tasted sour were considered as acids (for example-citric acid, acetic acid, oxalic acid), and substances that tasted bitter were considered as bases. However, this proved to be wrong later on when scientists were unable to explain the concept of acids and bases in a solvent other than water or aqueous solution.
However, since then many theories have come up explaining the acidic behaviour of acids and the basic properties of hydroxides in terms of their ability to yield hydrogen and hydroxide ions in solutions, such as the Arrhenius theory, Lewis Acid-Base theory, Lowry-Bronsted Acid-Base theory, Pearson’s concept and many other theories.
Arrhenius Theory
The Arrhenius theory was proposed by Swedish chemist Svante Arrhenius in 1884. Svante August Arrhenius was popularly known for his work in electrolytic dissociation and also for the famous Arrhenius equation. He was also awarded the Nobel prize in 1903. This theory was used to classify acids and bases on the basis of the type of ions they dissociate on dissolving in water. If a compound releases H+ after dissociation it is categorised as acid and if a compound releases OH– after dissociation it is categorised as base.
Arrhenius Acid
According to Arrhenius theory, an acid is a substance that dissociates to give hydrogen ions when dissolved in water. For example: When hydrogen chloride gas dissolves in water, it gives hydrogen ions,
The release of hydrogen ions increases the concentration of hydrogen ions in solution and makes the solution acidic.
Arrhenius Base
According to Arrhenius, base is a substance that dissolves to give hydroxide ions when dissolved in water.
For example: When sodium hydroxide dissolves in water it releases hydroxide ion
The release of hydroxide ion increases the concentration in the solution and makes the solution basic.
Advantages of Arrhenius Theory
The various advantages of Arrhenius theory are that it provides knowledge about:
- The properties of acids and bases
- The strength of acids and bases
- Concept of neutralisation and hydrolysis
Neutralisation
When an equal amount of acid and base reacts it forms a salt, which is neither acid nor base.

Limitations of Arrhenius Theory
- Arrhenius theory explains acids and bases in terms of their presence in aqueous solution and not as a substance. As such the theory is limited to the study of acids and bases in aqueous solution only and not applicable in gaseous and non-aqueous solutions.
- Arrhenius theory is applicable only to acids having the formula HA. For example: HCl, HBr. It is not applicable for SO2, CO2, and AlCl3.
- Arrhenius theory is only applicable to bases having the formula BOH. For example: NaOH, KOH. It is not applicable for Na2CO3, NH3 and pyridine. In fact, the need to prove the relationship between base and OH ion led to the proposal of the formula NH4OH as base of ammonia in water, the actual formula being NH3.
- According to this theory, solvents do not play any role in deciding the nature of acids and bases. For example, hydrochloric acid is strong when dissolved in water but it is weak when dissolved in benzene.
- This theory fails to explain the properties of acids and bases in any solvents )like benzene or acetone) or gases, other than water.
- According to Arrhenius, all salts should produce solutions that are neither acidic nor basic. However, there are some exceptions to this and Arrhenius theory fails to explain this For example:
- Equal amounts of HCL and ammonia yield a slightly acidic solution
- Equal amounts of acetic acid and sodium hydroxide yield a slightly basic solution
- According to this theory, hydrogen ions release in the aqueous solution and remain there, increasing the concentration of hydrogen ions in the solution. However, this is not true because hydrogen can never exist freely as hydrogen ions. It exists as hydronium ion (H3O+) .
In what Form Hydrogen exists in Water?
Hydrogen does not exist freely as hydrogen ions (H+) in water. It exists as an aqueous cation known as the hydronium ion (H3O+). It is a type of oxonium ion produced by the protonation of water. Its conjugate base is water.
Related Links:
Conclusion
Arrhenius theory is only applicable when a substance is dissolved in water. It considers only those substances as acids, which release hydrogen ions in aqueous solutions and only those substances as bases, which releases hydroxide ions in aqueous solutions. The theory fails to explain the exception of neutralisation. The Arrhenius concept was the very first inference that was drawn, and thus it had several flaws to speak of as it was thought of so long ago when other notions with regard to chemistry had not yet come into play. Since not much was known, Arrhenius based his ideas on water, rather, on protons alone, disregarding electrons, unlike Lewis in later years.
As the world around us evolves, so do natural phenomena, so do the reasons behind them, or in other words, science. Ideas, thought processes, and inferences alter and shift with the ages, and we find new theories because the previous ones failed to explain certain aspects of the phenomena. The first theories often cause a stir among the populace, in fact, Arrhenius was a Nobel laureate, despite the Bronsted Lowry and Lewis theory being more feasible as the former theory was considered too specific in 1923.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out