The chemical bond shared among the atoms in the molecules is described by the molecular orbital hypothesis. It postulates that two atomic orbitals must overlap to establish a molecular bond. The overlapping of more than one orbital results in the emergence of molecular orbitals. Bonding and antibonding orbitals are the two categories of molecular orbitals. Bond electrons make up bonding molecular orbitals or BMOs. The electrons form a covalent linkage when they combine. On the other hand, antibonding molecular orbitals don’t contribute to bonding, and hence they are found located external to the bond.
What are molecular orbitals?
The molecular orbital theory (MOT) explains the electron distribution in molecules in the same manner that atomic orbitals describe the electron distribution in atoms. Electrons surrounding atoms in molecules have discrete energy levels. A molecular orbital is the region of space where a molecule’s valence electron is most commonly present. Similar to atomic orbitals, a molecular orbital is filled when it has 2 electrons with opposite spins. There are two major types of molecular orbitals. They are bonding and antibonding orbitals. Let’s now move on to discuss these types of molecular orbitals.
Check out the complete UPSC Syllabus
Different types of molecular orbitals
Based on the atomic orbital interactions, molecular orbitals are classified into three categories.
- Bonding molecular orbitals
- Antibonding molecular orbitals
- Non-bonding molecular orbitals
This article concerns the bonding and antibonding orbitals of molecules and their differences. Let’s discuss them in more detail.
Bonding molecular orbitals (BMO)
Bonding interactions are constructive interactions among atomic orbitals. The BMO is created when 2 atomic orbitals from 2 separate atoms overlap. This overlapping causes both the atomic orbitals to mix and produce these molecular orbitals. Both the atomic orbitals must have equivalent energies and perfect symmetry to be combined.
Bonding molecular orbitals or bonding MOs have a higher density of electrons than ABMOs. The bonding MOs are of lower energy than the parent atomic orbitals that unite to form them. They contribute to forming chemical bonds. Since lower energy levels signal more stability, these bonding MOs have high stability. The bonding molecular orbitals help determine molecular geometry.
Antibonding molecular orbitals
Molecular orbitals where the electrons are exteriorly located away from the space between the 2 atomic nuclei are known as antibonding orbitals. Because antibonding orbital electrons are mostly always located externally to the atomic nuclei, they diminish a molecule’s stability. As a result, antibonding molecular orbitals have a lower electron density than bonding MOs or bonding molecular orbitals.
The energy of ABMOs or antibonding molecular orbitals is higher than the bonding MOs and the atomic orbitals. As a result, compounds with electrons in antibonding molecular orbitals have reduced stability. However, in certain compounds that are stable, there are few or no antibonding orbital electrons. Additionally, the molecular geometry or shape is not determined by the spatial configuration of ABMOs.
Visit to know more about UPSC Preparation Books
Difference between bonding and antibonding molecular orbitals
The difference between bonding and antibonding molecular orbitals is given below.
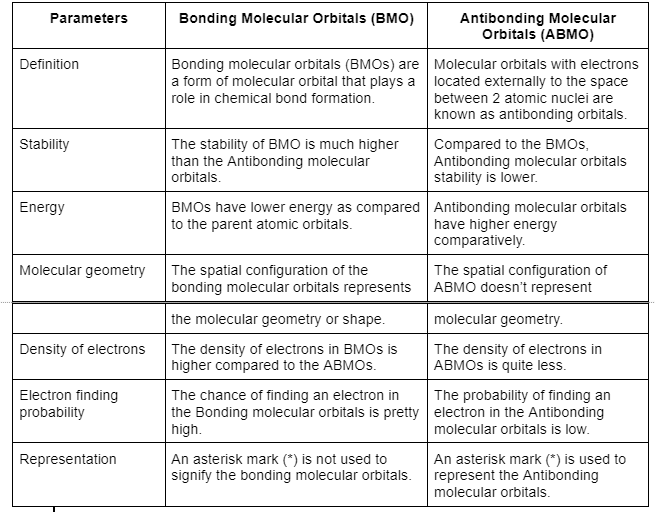
Also see UPSC Notes
Conclusion
The molecular orbital model describes how two atoms create a chemical linkage by overlapping their atomic orbitals. Molecular orbitals are formed when atomic orbitals are mixed to produce new orbitals. Bonding and antibonding orbitals are two types of molecular orbitals. It is important to understand both molecular orbitals in detail to know their differences. There are several differences between bonding and antibonding molecular orbitals. One such distinction is that the bonding molecular orbitals depict a molecule’s shape, but antibonding molecular orbitals can’t aid in determining a molecule’s shape. In addition, antibonding molecular orbitals have lower stability than bonding molecular orbitals.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











