Phosphate Formula
Phosphate is also known as orthophosphate or phosphate ion. It is a conjugate base comprising hydrogen phosphate and a trivalent inorganic anion.
It can be found in bones, DNA, and teeth in the human body. Many phosphate minerals include phosphorus. Morocco is the world’s largest phosphate exporter and producer.
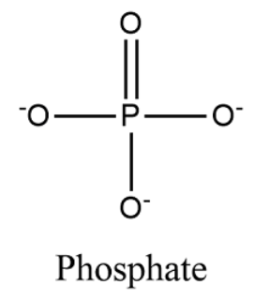
The phosphate ion has a molar mass of 94.97 g/mol and is made up of a phosphorus atom in the centre surrounded by four oxygen atoms in a tetrahedral configuration. It is the conjugate base of the hydrogen phosphate ion, which is the conjugate base of the dihydrogen phosphate ion, which is the conjugate base of orthophosphoric acid.
Phosphate has the empirical formula PO43- . It has a molecular mass of 94.9714 g/mol and is a polyatomic ion.
The structure of phosphate is as follows;
Phosphates are classified as follows:
(1) Primary phosphates – liquid crystallised
(2) Secondary phosphates are formed when primary phosphates are modified
(3) Fine-grained rock phosphates – produced at low temperatures from phosphorus-bearing organic material
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




